Fc or not Fc; that is the question: Antibody Fc-receptor interactions are key to universal influenza vaccine design
- PMID: 28332900
- PMCID: PMC5489290
- DOI: 10.1080/21645515.2017.1290018
Fc or not Fc; that is the question: Antibody Fc-receptor interactions are key to universal influenza vaccine design
Abstract
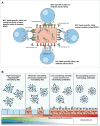
A universal vaccine that provides long-lasting protection from both epidemic and pandemic influenza viruses remains the "holy grail" of influenza vaccine research. Though virus neutralization assays are the current benchmark of measuring vaccine effectiveness, it is clear that Fc-receptor functions can drastically improve the effectiveness of antibodies and vaccines in vivo. Antibodies that kill virus-infected cells and/or elicit an antiviral environment, termed antibody-dependent cellular cytotoxicity (ADCC)-mediating antibodies, provide a link between the innate and adaptive immune response. New technologies allowing the rapid isolation and characterization of monoclonal antibodies (mAb) have yielded a plethora of mAbs which target conserved regions of influenza virus, such as the hemagglutinin (HA) stem region. Many such mAbs have been used to gain a better understanding of Fc-receptor functions in vivo. In parallel, several studies have characterized the induction of polyclonal ADCC following influenza vaccination and infection in humans. Taken together, these studies suggest that ADCC-mediating antibodies (ADCC-Abs) significantly contribute to host immunity against influenza virus and may be a mechanism to exploit for rational vaccine and therapeutic design. We discuss recent research on influenza-specific ADCC and potential future avenues to extend our understanding.
Keywords: antibody-dependent cellular cytotoxicity; influenza; monoclonal antibodies; universal vaccine.
Figures
References
-
- WHO Influenza (Seasonal), Fact sheet. http://www.who.int/mediacentre/factsheets/fs211/en/, 2016
-
- Molinari NA, Ortega-Sanchez IR, Messonnier ML, Thompson WW, Wortley PM, Weintraub E, Bridges CB. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine 2007; 25:5086-96; PMID:17544181; https://doi.org/ 10.1016/j.vaccine.2007.03.046 - DOI - PubMed
-
- Radin JM, Hawksworth AW, Myers CA, Ricketts MN, Hansen EA, Brice GT. Influenza vaccine effectiveness: Maintained protection throughout the duration of influenza seasons 2010-2011 through 2013-2014. Vaccine 2016; 34:3907-12; PMID:27265447; https://doi.org/ 10.1016/j.vaccine.2016.05.034 - DOI - PubMed
-
- Bedford T, Suchard MA, Lemey P, Dudas G, Gregory V, Hay AJ, McCauley JW, Russell CA, Smith DJ, Rambaut A. Integrating influenza antigenic dynamics with molecular evolution. Elife 2014; 3:e01914; https://doi.org/ 10.7554/eLife.01914 - DOI - PMC - PubMed
-
- Impagliazzo A, Milder F, Kuipers H, Wagner MV, Zhu X, Hoffman RM, van Meersbergen R, Huizingh J, Wanningen P, Verspuij J, et al.. A stable trimeric influenza hemagglutinin stem as a broadly protective immunogen. Science 2015; 349:1301-6; PMID:26303961; https://doi.org/ 10.1126/science.aac7263 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
