Anti-Melanogenic Properties of Greek Plants. A Novel Depigmenting Agent from Morus alba Wood
- PMID: 28333105
- PMCID: PMC6154579
- DOI: 10.3390/molecules22040514
Anti-Melanogenic Properties of Greek Plants. A Novel Depigmenting Agent from Morus alba Wood
Abstract
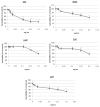
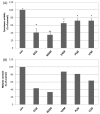
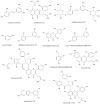
In therapeutic interventions associated with melanin hyperpigmentation, tyrosinase is regarded as a target enzyme as it catalyzes the rate-limiting steps in mammalian melanogenesis. Since many known agents have been proven to be toxic, there has been increasing impetus to identify alternative tyrosinase inhibitors, especially from natural sources. In this study, we investigated 900 extracts from Greek plants for potential tyrosinase inhibitive properties. Among the five most potent extracts, the methanol extract of Morus alba wood (MAM) demonstrated a significant reduction in intracellular tyrosinase and melanin content in B16F10 melanoma cells. Bioassay-guided isolation led to the acquisition of twelve compounds: oxyresveratrol (1), kuwanon C (2), mulberroside A (3), resorcinol (4), dihydrooxyresveratol (5), trans-dihydromorin (6), 2,4,3'-trihydroxydihydrostilbene (7), kuwanon H (8), 2,4-dihydroxybenzaldehyde (9), morusin (10), moracin M (11) and kuwanon G (12). Among these, 2,4,3'-trihydroxydihydrostilbene (7) is isolated for the first time from Morus alba and constitutes a novel potent tyrosinase inhibitor (IC50 0.8 ± 0.15). We report here for the first time dihydrooxyresveratrol (5) as a potent natural tyrosinase inhibitor (IC50 0.3 ± 0.05). Computational docking analysis indicated the binding modes of six tyrosinase inhibitors with the aminoacids of the active centre of tyrosinase. Finally, we found both MAM extract and compounds 1, 6 and 7 to significantly suppress in vivo melanogenesis during zebrafish embryogenesis.
Keywords: 2,4,3′-trihydroxydihydrostilbene; B16F10 melanoma cells; Greek flora; Morus alba; computational docking analysis; dihydroxyresveratrol; melanogenesis; tyrosinase inhibition; zebrafish.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Characterization of Melanogenesis Inhibitory Constituents of Morus alba Leaves and Optimization of Extraction Conditions Using Response Surface Methodology.Molecules. 2015 May 14;20(5):8730-41. doi: 10.3390/molecules20058730. Molecules. 2015. PMID: 26007176 Free PMC article.
-
Identification of Anti-Melanogenesis Constituents from Morus alba L. Leaves.Molecules. 2018 Oct 8;23(10):2559. doi: 10.3390/molecules23102559. Molecules. 2018. PMID: 30297610 Free PMC article.
-
Mulberroside F isolated from the leaves of Morus alba inhibits melanin biosynthesis.Biol Pharm Bull. 2002 Aug;25(8):1045-8. doi: 10.1248/bpb.25.1045. Biol Pharm Bull. 2002. PMID: 12186407
-
Moraceae Plants with Tyrosinase Inhibitory Activity: A Review.Mini Rev Med Chem. 2017;17(2):108-121. doi: 10.2174/1389557516666160609071854. Mini Rev Med Chem. 2017. PMID: 27292779 Review.
-
Small-Molecule Tyrosinase Inhibitors for Treatment of Hyperpigmentation.Molecules. 2025 Feb 8;30(4):788. doi: 10.3390/molecules30040788. Molecules. 2025. PMID: 40005101 Free PMC article. Review.
Cited by
-
A Zebrafish Embryo as an Animal Model for the Treatment of Hyperpigmentation in Cosmetic Dermatology Medicine.Medicina (Kaunas). 2018 May 25;54(3):35. doi: 10.3390/medicina54030035. Medicina (Kaunas). 2018. PMID: 30344266 Free PMC article. Review.
-
The structure-activity relationship review of the main bioactive constituents of Morus genus plants.J Nat Med. 2020 Mar;74(2):331-340. doi: 10.1007/s11418-019-01383-8. Epub 2020 Jan 2. J Nat Med. 2020. PMID: 31897975 Free PMC article. Review.
-
Phytochemical Study and In Vitro Screening Focusing on the Anti-Aging Features of Various Plants of the Greek Flora.Antioxidants (Basel). 2021 Jul 28;10(8):1206. doi: 10.3390/antiox10081206. Antioxidants (Basel). 2021. PMID: 34439454 Free PMC article.
-
Comprehensive overview of different medicinal parts from Morus alba L.: chemical compositions and pharmacological activities.Front Pharmacol. 2024 Apr 17;15:1364948. doi: 10.3389/fphar.2024.1364948. eCollection 2024. Front Pharmacol. 2024. PMID: 38694910 Free PMC article. Review.
-
Exploring the therapeutic and anti-tumor properties of morusin: a review of recent advances.Front Mol Biosci. 2023 May 9;10:1168298. doi: 10.3389/fmolb.2023.1168298. eCollection 2023. Front Mol Biosci. 2023. PMID: 37228582 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

