Targeting 3-phosphoinositide-dependent protein kinase 1 associated with drug-resistant renal cell carcinoma using new oridonin analogs
- PMID: 28333136
- PMCID: PMC5386527
- DOI: 10.1038/cddis.2017.121
Targeting 3-phosphoinositide-dependent protein kinase 1 associated with drug-resistant renal cell carcinoma using new oridonin analogs
Abstract
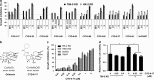
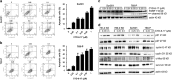
The current agents used for renal cell carcinoma (RCC) only exhibit the moderate response rate among patients. Development of drug resistance eventually fuels the need of either more potent drugs or new drugs to target the resistant pathways. Oridonin is a diterpenoid isolated from the Chinese medicinal herb Rabdosia rubescens and has been shown to have antitumor activities in many cancers. We previously developed new synthetic methodologies to modify structurally diversified diterpenoids and designed a series of nitrogen-enriched oridonin analogs. In this study, we screened a variety of oridonin analogs based on their cytotoxicity using MTT assay and identify the most potent candidate, namely, CYD-6-17. CYD-6-17 exhibited a high potency to inhibit the in vitro growth of several drug-resistant RCC cells as well as endothelial cells stimulated by tumor cells at nanomolar range. Delivery of CYD-6-17 significantly inhibited RCC tumor growth using xenograft model. Mechanistically, it targeted the 3-phosphoinositide-dependent protein kinase 1 gene that appeared to be a potent regulator of AKT and was associated with patient survival after targeted therapies. This offers a new rational therapeutic regimen of CYD-6-17 to drug-resistant RCC based on its novel mechanism of action.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Rini BI, Atkins MB. Resistance to targeted therapy in renal-cell carcinoma. Lancet Oncol 2009; 10: 992–1000. - PubMed
-
- Hsiao WL, Liu L. The role of traditional Chinese herbal medicines in cancer therapy--from TCM theory to mechanistic insights. Planta Medica 2010; 76: 1118–1131. - PubMed
-
- Liu Y, Liu YZ, Zhang RX, Wang X, Meng ZJ, Huang J et al. Oridonin inhibits the proliferation of human osteosarcoma cells by suppressing Wnt/beta-catenin signaling. Int J Oncol 2014; 45: 795–803. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

