The cryo-thermal therapy eradicated melanoma in mice by eliciting CD4+ T-cell-mediated antitumor memory immune response
- PMID: 28333145
- PMCID: PMC5386530
- DOI: 10.1038/cddis.2017.125
The cryo-thermal therapy eradicated melanoma in mice by eliciting CD4+ T-cell-mediated antitumor memory immune response
Abstract
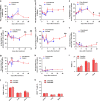
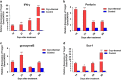
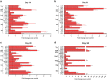
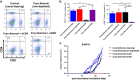
Tumor metastasis is a major concern in tumor therapy. In our previous studies, a novel tumor therapeutic modality of the cryo-thermal therapy has been presented, highlighting its effect on the suppression of distal metastasis and leading to long-term survival in 4T1 murine mammary carcinoma model. To demonstrate the therapeutic efficacy in other aggressive tumor models and further investigate the mechanism of long-term survival induced, in this study, spontaneous metastatic murine B16F10 melanoma model was used. The cryo-thermal therapy induced regression of implanted melanoma and prolonged long-term survival while inhibiting lung metastasis. It also promoted the activation of CD4+ CD25- conventional T cells, while reduced the percentage of CD4+ CD25+ regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs) in the spleen, lung and blood. Furthermore, the cryo-thermal therapy enhanced the cytolytic function of CD8+ T cells and induced differentiation of CD8+ T cells into memory stem T cell (TSCM), and differentiation of CD4+ T cells into dominant CD4-CTL, Th1 and Tfh subsets in the spleen for 90 days after the treatment. It was found that good therapeutic effect was mainly dependent on CD4+ T cells providing a durable memory antitumor immune response. At the same time, significant increase of serum IFN-γ was also observed to provide an ideal microenvironment of antitumor immunity. Further study showed that the rejection of re-challenge of B16F10 but not GL261 tumor in the treated mice in 45 or 60 days after the treatment, implied a strong systemic and melanoma-specific memory antitumor immunity induced by the treatment. Thus the cryo-thermal therapy would be considered as a new therapeutic strategy to prevent tumor recurrence and metastasis with potential clinical applications in the near future.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

