The Endoplasmic Reticulum Chaperone GRP78/BiP Modulates Prion Propagation in vitro and in vivo
- PMID: 28333162
- PMCID: PMC5363067
- DOI: 10.1038/srep44723
The Endoplasmic Reticulum Chaperone GRP78/BiP Modulates Prion Propagation in vitro and in vivo
Abstract
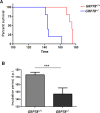
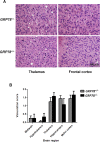
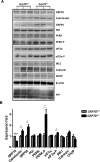
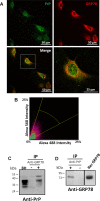
Prion diseases are fatal neurodegenerative disorders affecting several mammalian species, characterized by the accumulation of the misfolded form of the prion protein, which is followed by the induction of endoplasmic reticulum (ER) stress and the activation of the unfolded protein response (UPR). GRP78, also called BiP, is a master regulator of the UPR, reducing ER stress levels and apoptosis due to an enhancement of the cellular folding capacity. Here, we studied the role of GRP78 in prion diseases using several in vivo and in vitro approaches. Our results show that a reduction in the expression of this molecular chaperone accelerates prion pathogenesis in vivo. In addition, we observed that prion replication in cell culture was inversely related to the levels of expression of GRP78 and that both proteins interact in the cellular context. Finally, incubation of PrPSc with recombinant GRP78 led to the dose-dependent reduction of protease-resistant PrPSc in vitro. Our results uncover a novel role of GRP78 in reducing prion pathogenesis, suggesting that modulating its levels/activity may offer a novel opportunity for designing therapeutic approaches for these diseases. These findings may also have implications for other diseases involving the accumulation of misfolded proteins.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

Similar articles
-
Prion disease is accelerated in mice lacking stress-induced heat shock protein 70 (HSP70).J Biol Chem. 2019 Sep 13;294(37):13619-13628. doi: 10.1074/jbc.RA118.006186. Epub 2019 Jul 18. J Biol Chem. 2019. PMID: 31320473 Free PMC article.
-
The disulfide isomerase Grp58 is a protective factor against prion neurotoxicity.J Neurosci. 2005 Mar 16;25(11):2793-802. doi: 10.1523/JNEUROSCI.4090-04.2005. J Neurosci. 2005. PMID: 15772339 Free PMC article.
-
Molecular mechanisms of neurotoxicity of pathological prion protein.Curr Mol Med. 2004 Jun;4(4):397-403. doi: 10.2174/1566524043360654. Curr Mol Med. 2004. PMID: 15354870 Review.
-
Prion-Associated Neurodegeneration Causes Both Endoplasmic Reticulum Stress and Proteasome Impairment in a Murine Model of Spontaneous Disease.Int J Mol Sci. 2021 Jan 5;22(1):465. doi: 10.3390/ijms22010465. Int J Mol Sci. 2021. PMID: 33466523 Free PMC article.
-
Stressing out the ER: a role of the unfolded protein response in prion-related disorders.Curr Mol Med. 2006 Feb;6(1):37-43. doi: 10.2174/156652406775574578. Curr Mol Med. 2006. PMID: 16472111 Free PMC article. Review.
Cited by
-
AR12 increases BAG3 expression which is essential for Tau and APP degradation via LC3-associated phagocytosis and macroautophagy.Aging (Albany NY). 2022 Oct 13;14(20):8221-8242. doi: 10.18632/aging.204337. Epub 2022 Oct 13. Aging (Albany NY). 2022. PMID: 36227739 Free PMC article.
-
Clearance of variant Creutzfeldt-Jakob disease prions in vivo by the Hsp70 disaggregase system.Brain. 2022 Sep 14;145(9):3236-3249. doi: 10.1093/brain/awac144. Brain. 2022. PMID: 35446941 Free PMC article.
-
Deregulation of mTORC1-TFEB axis in human iPSC model of GBA1-associated Parkinson's disease.Front Neurosci. 2023 Jun 2;17:1152503. doi: 10.3389/fnins.2023.1152503. eCollection 2023. Front Neurosci. 2023. PMID: 37332877 Free PMC article.
-
Prion disease is accelerated in mice lacking stress-induced heat shock protein 70 (HSP70).J Biol Chem. 2019 Sep 13;294(37):13619-13628. doi: 10.1074/jbc.RA118.006186. Epub 2019 Jul 18. J Biol Chem. 2019. PMID: 31320473 Free PMC article.
-
Butyrylcholinesterase in SH-SY5Y human neuroblastoma cells.Neurotoxicology. 2022 May;90:1-9. doi: 10.1016/j.neuro.2022.02.006. Epub 2022 Feb 18. Neurotoxicology. 2022. PMID: 35189179 Free PMC article.
References
-
- Soto C. & Saborio G. P. Prions: disease propagation and disease therapy by conformational transmission. Trends Mol. Med. 7, 109–114 (2001). - PubMed
-
- Halliday M. & Mallucci G. R. Targeting the unfolded protein response in neurodegeneration: A new approach to therapy. 76 Pt A, 169–174 (2014). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

