Protein aggregation in Ehrlichia chaffeensis during infection of mammalian cells
- PMID: 28333306
- PMCID: PMC5399918
- DOI: 10.1093/femsle/fnx059
Protein aggregation in Ehrlichia chaffeensis during infection of mammalian cells
Abstract
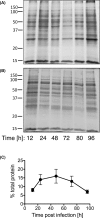
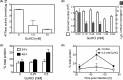
Ehrlichia chaffeensis is an obligatory intracellular pathogen transmitted through infected ticks to humans and other vertebrates. We investigated the extent of protein aggregation in E. chaffeensis during infection of canine macrophage cell line, DH82. We discovered that the size of the aggregated fraction of E. chaffeensis proteins increased during the first 48 h post infection. We also incubated the infected cells with guanidinium chloride (GuHCl), a known inhibitor of the protein-disaggregating molecular chaperone ClpB. Up to 0.5 mM GuHCl had no impact on the host cells, whereas the viability of the pathogen was reduced by ∼60% in the presence of the inhibitor. Furthermore, we found that the size of the aggregated protein fraction in E. chaffeensis increased significantly in cultures supplemented with 0.5 mM GuHCl, which also resulted in the preferential accumulation of ClpB with the aggregated proteins. Altogether, our results suggest that an exposure of E. chaffeensis to the stressful environment of a host cell results in an increased aggregation of the pathogen's proteins, which is exacerbated upon inhibition of ClpB. Our studies establish a link between protein quality control and pathogen survival during infection of a host.
Keywords: ClpB; Ehrlichia chaffeensis; host–pathogen interaction; molecular chaperone; protein aggregation; protein folding.
© FEMS 2017. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures

References
-
- Elsworth B, Matthews K, Nie CQ et al. . PTEX is an essential nexus for protein export in malaria parasites. Nature 2014;511:587–91. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

