In vivo multiphoton-microscopy of picosecond-laser-induced optical breakdown in human skin
- PMID: 28333369
- PMCID: PMC5513776
- DOI: 10.1002/lsm.22655
In vivo multiphoton-microscopy of picosecond-laser-induced optical breakdown in human skin
Abstract
Importance: Improvements in skin appearance resulting from treatment with fractionated picosecond-lasers have been noted, but optimizing the treatment efficacy depends on a thorough understanding of the specific skin response. The development of non-invasive laser imaging techniques in conjunction with laser therapy can potentially provide feedback for guidance and optimizing clinical outcome.
Objective: The purpose of this study was to demonstrate the capability of multiphoton microscopy (MPM), a high-resolution, label-free imaging technique, to characterize in vivo the skin response to a fractionated non-ablative picosecond-laser treatment.
Design, setting, and participants: Two areas on the arm of a volunteer were treated with a fractionated picosecond laser at the Dermatology Clinic, UC Irvine. The skin response to treatment was imaged in vivo with a clinical MPM-based tomograph at 3 hours and 24 hours after treatment and seven additional time points over a 4-week period.
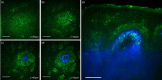
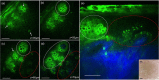
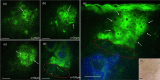
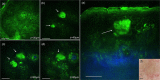
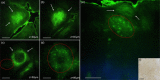
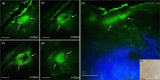
Main outcomes and measures: MPM revealed micro-injuries present in the epidermis. Pigmented cells were particularly damaged in the process, suggesting that melanin is likely the main absorber for laser induced optical breakdown.
Results: Damaged individual cells were distinguished as early as 3 hours post pico-laser treatment with the 532 nm wavelength, and 24 hours post-treatment with both 532 and 1064 nm wavelengths. At later time points, clusters of cellular necrotic debris were imaged across the treated epidermis. After 24 hours of treatment, inflammatory cells were imaged in the proximity of epidermal micro-injuries. The epidermal injuries were exfoliated over a 4-week period.
Conclusions and relevance: This observational and descriptive pilot study demonstrates that in vivo MPM imaging can be used non-invasively to provide label-free contrast for describing changes in human skin following a fractionated non-ablative laser treatment. The results presented in this study represent the groundwork for future longitudinal investigations on an expanded number of subjects to understand the response to treatment in different skin types with different laser parameters, critical factors in optimizing treatment outcome. Lasers Surg. Med. 49:555-562, 2017. © 2017 Wiley Periodicals, Inc.
Keywords: in vivo imaging; laser induced optical breakdown; noninvasive multiphoton microscopy.
© 2017 Wiley Periodicals, Inc.
Figures
References
-
- Tanghetti EA. The histology of skin treated with a picosecond alexandrite laser and a fractional lens array. Lasers Surg Med 2016; 48(7):646–652. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

