Ventricular arrhythmias in patients with newly diagnosed nonischemic cardiomyopathy: Insights from the PROLONG study
- PMID: 28333373
- PMCID: PMC6490321
- DOI: 10.1002/clc.22706
Ventricular arrhythmias in patients with newly diagnosed nonischemic cardiomyopathy: Insights from the PROLONG study
Abstract
Background: Patients with nonischemic cardiomyopathy (NICM) reportedly have low incidence of appropriate shocks from wearable cardioverter-defibrillators (WCDs). A recent study questions the benefit from primary preventive implantation of implantable cardioverter-defibrillators in NICM. We therefore analyzed a subgroup of patients with NICM from the PROLONG study.
Hypothesis: Patients with newly diagnosed NICM show a risk for ventricular tachyarrhythmia.
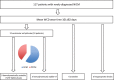
Methods: The PROLONG study included 167 patients with newly diagnosed heart failure and left ventricular ejection fraction (LVEF) ≤35% with a WCD. Patients with NICM were identified and included in this analysis.
Results: 117 patients presented with NICM. Sixty-five (55%) were male; mean age was 51 ± 15 years. Mean LVEF at diagnosis was 23% ± 7%. Mean follow-up was 11 ± 10 months. Mean WCD wear time was 101 ± 82 days; mean wear time per day was 21.4 ± 4.5 hours. Overall, 12 ventricular arrhythmias occurred in 10 (9%) patients (6 DCM, 4 PPCM). Nine appropriate WCD shocks for hemodynamically unstable ventricular tachycardia/fibrillation in 8 (7%) patients were observed. Two patients presented sustained hemodynamically stable ventricular tachycardia for >30 minutes detected by the WCD, but withheld WCD therapy.
Conclusions: Patients with newly diagnosed NICM and LVEF ≤35% show an elevated risk of ventricular tachycardia/fibrillation during initiation and optimization of heart failure therapy. To prevent sudden cardiac death, WCD should be considered in patients with newly diagnosed NICM with severely reduced LVEF.
Keywords: Nonischemic Cardiomyopathy; Sudden Cardiac Death; Ventricular Arrhythmia; Wearable Cardioverter-Defibrillator.
© 2017 Wiley Periodicals, Inc.
Conflict of interest statement
DD received lecture honoraria and travel support from Zoll. CV received lecture honoraria, travel support, and a research grant from Zoll. The authors declare no other potential conflicts of interest.
Figures

References
-
- Kutyifa V, Moss AJ, Klein H, et al. Use of the wearable cardioverter‐defibrillator in high‐risk cardiac patients: data from the Prospective Registry of Patients Using the Wearable Cardioverter Defibrillator (WEARIT‐II Registry). Circulation. 2015;132:1613–1619. - PubMed
-
- Singh M, Wang NC, Jain S, et al. Utility of the wearable cardioverter‐defibrillator in patients with newly diagnosed cardiomyopathy: a decade‐long single‐center experience. J Am Coll Cardiol. 2015;66:2607–2613. - PubMed
-
- Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC [published correction appears in Eur Heart J. 2016;pii:ehw383]. Eur Heart J . 2016;37:2129–2200. - PubMed
-
- Priori SG, Blomström‐Lundqvist C, Mazzanti A, et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J . 2015;36:2793–2867. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

