Regulation of Coronary Blood Flow
- PMID: 28333376
- PMCID: PMC5966026
- DOI: 10.1002/cphy.c160016
Regulation of Coronary Blood Flow
Abstract
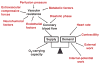
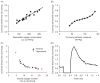
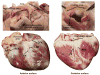
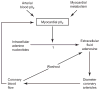
The heart is uniquely responsible for providing its own blood supply through the coronary circulation. Regulation of coronary blood flow is quite complex and, after over 100 years of dedicated research, is understood to be dictated through multiple mechanisms that include extravascular compressive forces (tissue pressure), coronary perfusion pressure, myogenic, local metabolic, endothelial as well as neural and hormonal influences. While each of these determinants can have profound influence over myocardial perfusion, largely through effects on end-effector ion channels, these mechanisms collectively modulate coronary vascular resistance and act to ensure that the myocardial requirements for oxygen and substrates are adequately provided by the coronary circulation. The purpose of this series of Comprehensive Physiology is to highlight current knowledge regarding the physiologic regulation of coronary blood flow, with emphasis on functional anatomy and the interplay between the physical and biological determinants of myocardial oxygen delivery. © 2017 American Physiological Society. Compr Physiol 7:321-382, 2017.
Copyright © 2017 John Wiley & Sons, Inc.
Figures
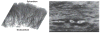



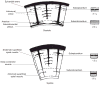
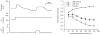

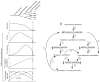
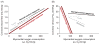


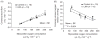

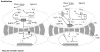
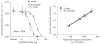
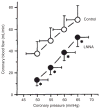







References
-
- Ahmed A, Waters CM, Leffler CW, Jaggar JH. Ionic mechanisms mediating the myogenic response in newborn porcine cerebral arteries. Am J Physiol Heart Circ Physiol. 2004;287:H2061–H2069. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

