Establishing a Timeline to Discontinue Routine Testing of Asymptomatic Pregnant Women for Zika Virus Infection - American Samoa, 2016-2017
- PMID: 28333910
- PMCID: PMC5657887
- DOI: 10.15585/mmwr.mm6611a5
Establishing a Timeline to Discontinue Routine Testing of Asymptomatic Pregnant Women for Zika Virus Infection - American Samoa, 2016-2017
Abstract
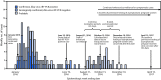
The first patients with laboratory-confirmed cases of Zika virus disease in American Samoa had symptom onset in January 2016 (1). In response, the American Samoa Department of Health (ASDoH) implemented mosquito control measures (1), strategies to protect pregnant women (1), syndromic surveillance based on electronic health record (EHR) reports (1), Zika virus testing of persons with one or more signs or symptoms of Zika virus disease (fever, rash, arthralgia, or conjunctivitis) (1-3), and routine testing of all asymptomatic pregnant women in accordance with CDC guidance (2,3). All collected blood and urine specimens were shipped to the Hawaii Department of Health Laboratory for Zika virus testing and to CDC for confirmatory testing. Early in the response, collection and testing of specimens from pregnant women was prioritized over the collection from symptomatic nonpregnant patients because of limited testing and shipping capacity. The weekly numbers of suspected Zika virus disease cases declined from an average of six per week in January-February 2016 to one per week in May 2016. By August, the EHR-based syndromic surveillance (1) indicated a return to pre-outbreak levels. The last Zika virus disease case detected by real-time, reverse transcription-polymerase chain reaction (rRT-PCR) occurred in a patient who had symptom onset on June 19, 2016. In August 2016, ASDoH requested CDC support in assessing whether local transmission had been reduced or interrupted and in proposing a timeline for discontinuation of routine testing of asymptomatic pregnant women. An end date (October 15, 2016) was determined for active mosquito-borne transmission of Zika virus and a timeline was developed for discontinuation of routine screening of asymptomatic pregnant women in American Samoa (conception after December 10, 2016, with permissive testing for asymptomatic women who conceive through April 15, 2017).
Figures
References
-
- CDC. Guidance for U.S. laboratories testing for Zika virus infection. Atlanta, GA: US Department of Health and Human Services, CDC; 2016. https://www.cdc.gov/zika/laboratories/lab-guidance.html
-
- CDC. Interim CDC Zika response plan. Atlanta, GA: US Department of Health and Human Services, CDC; 2016. https://www.cdc.gov/zika/pdfs/zika-draft-interim-conus-plan.pdf
-
- Petersen EE, Meaney-Delman D, Neblett-Fanfair R, et al. Update: interim guidance for preconception counseling and prevention of sexual transmission of Zika virus for persons with possible Zika virus exposure—United States, September 2016. MMWR Morb Mortal Wkly Rep 2016;65:1077–81. 10.15585/mmwr.mm6539e1 - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

