Synthesis and bioevaluation of N,4-diaryl-1,3-thiazole-2-amines as tubulin inhibitors with potent antiproliferative activity
- PMID: 28333984
- PMCID: PMC5363846
- DOI: 10.1371/journal.pone.0174006
Synthesis and bioevaluation of N,4-diaryl-1,3-thiazole-2-amines as tubulin inhibitors with potent antiproliferative activity
Abstract
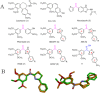
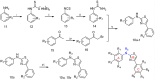
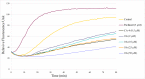
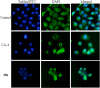
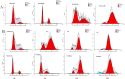
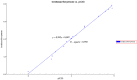
A series of N,4-diaryl-1,3-thiazole-2-amines containing three aromatic rings with an amino linker were designed and synthesized as tubulin inhibitors and evaluated for their antiproliferative activity in three human cancer cell lines. Most of the target compounds displayed moderate antiproliferative activity, and N-(2,4-dimethoxyphenyl)-4-(4-methoxyphenyl)-1,3-thiazol-2-amine (10s) was determined to be the most potent compound. Tubulin polymerization and immunostaining experiments revealed that 10s potently inhibited tubulin polymerization and disrupted tubulin microtubule dynamics in a manner similar to CA-4. Moreover, 10s effectively induced SGC-7901 cell cycle arrest at the G2/M phase in both concentration- and time-dependent manners. The molecular docking results revealed that 10s could bind to the colchicine binding site of tubulin.
Conflict of interest statement
Figures


References
-
- Stephens RE, Edds KT. Microtubules: structure, chemistry, and function. Physiol Rev. 1976; 56(4): 709–777. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

