Opposite effects of 5-HT/AKH and octopamine on the crop contractions in adult Drosophila melanogaster: Evidence of a double brain-gut serotonergic circuitry
- PMID: 28334024
- PMCID: PMC5363830
- DOI: 10.1371/journal.pone.0174172
Opposite effects of 5-HT/AKH and octopamine on the crop contractions in adult Drosophila melanogaster: Evidence of a double brain-gut serotonergic circuitry
Abstract
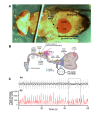
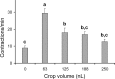
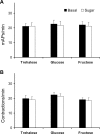
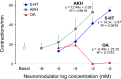
This study showed that in adult Drosophila melanogaster, the type of sugar-either present within the crop lumen or in the bathing solution of the crop-had no effect on crop muscle contraction. What is important, however, is the volume within the crop lumen. Electrophysiological recordings demonstrated that exogenous applications of serotonin on crop muscles increases both the amplitude and the frequency of crop contraction rate, while adipokinetic hormone mainly enhances the crop contraction frequency. Conversely, octopamine virtually silenced the overall crop activity. The present study reports for the first time an analysis of serotonin effects along the gut-brain axis in adult D. melanogaster. Injection of serotonin into the brain between the interocellar area shows that brain applications of serotonin decrease the frequency of crop activity. Based on our results, we propose that there are two different, opposite pathways for crop motility control governed by serotonin: excitatory when added in the abdomen (i.e., directly bathing the crop) and inhibitory when supplied within the brain (i.e., by injection). Finally, our results point to a double brain-gut serotonergic circuitry suggesting that not only the brain can affect gut functions, but the gut can also affect the central nervous system. On the basis of our results, and data in the literature, a possible mechanism for these two discrete serotonergic functions is suggested.
Conflict of interest statement
Figures



Similar articles
-
GnRH-Related Neurohormones in the Fruit Fly Drosophila melanogaster.Int J Mol Sci. 2021 May 10;22(9):5035. doi: 10.3390/ijms22095035. Int J Mol Sci. 2021. PMID: 34068603 Free PMC article. Review.
-
The imbalance of serotonergic circuitry impairing the crop supercontractile muscle activity and the mitochondrial morphology of PD PINK1B9Drosophila melanogaster are rescued by Mucuna pruriens.J Insect Physiol. 2018 Nov-Dec;111:32-40. doi: 10.1016/j.jinsphys.2018.10.007. Epub 2018 Oct 26. J Insect Physiol. 2018. PMID: 30393142
-
Adipokinetic hormone stimulates neurones in the insect central nervous system.J Exp Biol. 1995 Jun;198(Pt 6):1307-11. doi: 10.1242/jeb.198.6.1307. J Exp Biol. 1995. PMID: 7782718
-
The distribution and physiological effects of three evolutionarily and sequence-related neuropeptides in Rhodnius prolixus: Adipokinetic hormone, corazonin and adipokinetic hormone/corazonin-related peptide.Gen Comp Endocrinol. 2014 Jan 1;195:1-8. doi: 10.1016/j.ygcen.2013.10.012. Epub 2013 Oct 31. Gen Comp Endocrinol. 2014. PMID: 24184870
-
Regulation of insulin and adipokinetic hormone/glucagon production in flies.Wiley Interdiscip Rev Dev Biol. 2020 Mar;9(2):e360. doi: 10.1002/wdev.360. Epub 2019 Aug 4. Wiley Interdiscip Rev Dev Biol. 2020. PMID: 31379062 Free PMC article. Review.
Cited by
-
Drosophila TRPγ is required in neuroendocrine cells for post-ingestive food selection.Elife. 2022 Apr 13;11:e56726. doi: 10.7554/eLife.56726. Elife. 2022. PMID: 35416769 Free PMC article.
-
GnRH-Related Neurohormones in the Fruit Fly Drosophila melanogaster.Int J Mol Sci. 2021 May 10;22(9):5035. doi: 10.3390/ijms22095035. Int J Mol Sci. 2021. PMID: 34068603 Free PMC article. Review.
-
Aminergic Signaling Controls Ovarian Dormancy in Drosophila.Sci Rep. 2018 Feb 1;8(1):2030. doi: 10.1038/s41598-018-20407-z. Sci Rep. 2018. PMID: 29391447 Free PMC article.
-
Physiological characterization and regulation of the contractile properties of the mosquito ventral diverticulum (crop).J Insect Physiol. 2017 Nov;103:98-106. doi: 10.1016/j.jinsphys.2017.10.012. Epub 2017 Oct 28. J Insect Physiol. 2017. PMID: 29107658 Free PMC article.
-
The molecular interplay of the establishment of an infection - gene expression of Diaphorina citri gut and Candidatus Liberibacter asiaticus.BMC Genomics. 2021 Sep 21;22(1):677. doi: 10.1186/s12864-021-07988-2. BMC Genomics. 2021. PMID: 34544390 Free PMC article.
References
-
- Gorissen M, Flik G, Huising M. Peptides and proteins regulating food intake: a comparative view. Anim Biol. 2006;56: 447–473.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

