ANG1 treatment reduces muscle pathology and prevents a decline in perfusion in DMD mice
- PMID: 28334037
- PMCID: PMC5363921
- DOI: 10.1371/journal.pone.0174315
ANG1 treatment reduces muscle pathology and prevents a decline in perfusion in DMD mice
Abstract
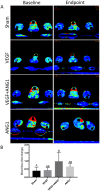
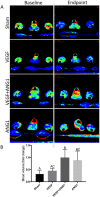
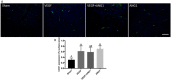
Vascular endothelial growth factor (VEGF) and other pro-angiogenic growth factors have been investigated to enhance muscle tissue perfusion and repair in Duchenne muscular dystrophy (DMD). Current understanding is limited by a lack of functional data following in vivo delivery of these growth factors. We previously used dynamic contrast-enhanced computed tomography to monitor disease progression in murine models of DMD, but no study to date has utilized this imaging technique to assess vascular therapy in a preclinical model of DMD. In the current study, we locally delivered VEGF and ANG1 alone or in combination to dystrophic hind limb skeletal muscle. Using functional imaging, we found the combination treatment as well as ANG1 alone prevented decline in muscle perfusion whereas VEGF alone had no effect compared to controls. These findings were validated histologically as demonstrated by increased alpha-smooth muscle actin-positive vessels in muscles that received either VEGF+ANG1 or ANG1 alone compared to the sham group. We further show that ANG1 alone slows progression of fibrosis compared to either sham or VEGF treatment. The findings from this study shed new light on the functional effects of vascular therapy and suggest that ANG1 alone may be a candidate therapy in the treatment of DMD.
Conflict of interest statement
Figures




References
-
- Loufrani L, Dubroca C, You D, Li Z, Levy B, Paulin D, et al. Absence of dystrophin in mice reduces NO-dependent vascular function and vascular density: total recovery after a treatment with the aminoglycoside gentamicin. Arterioscler Thromb Vasc Biol. 2004;24(4):671–6. 10.1161/01.ATV.0000118683.99628.42 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

