Differences in the photosynthetic plasticity of ferns and Ginkgo grown in experimentally controlled low [O2]:[CO2] atmospheres may explain their contrasting ecological fate across the Triassic-Jurassic mass extinction boundary
- PMID: 28334286
- PMCID: PMC5604595
- DOI: 10.1093/aob/mcx018
Differences in the photosynthetic plasticity of ferns and Ginkgo grown in experimentally controlled low [O2]:[CO2] atmospheres may explain their contrasting ecological fate across the Triassic-Jurassic mass extinction boundary
Abstract
Background and aims: Fluctuations in [CO 2 ] have been widely studied as a potential driver of plant evolution; however, the role of a fluctuating [O 2 ]:[CO 2 ] ratio is often overlooked. The present study aimed to investigate the inherent physiological plasticity of early diverging, extant species following acclimation to an atmosphere similar to that across the Triassic-Jurassic mass extinction interval (TJB, approx. 200 Mya), a time of major ecological change.
Methods: Mature plants from two angiosperm ( Drimys winteri and Chloranthus oldhamii ), two monilophyte ( Osmunda claytoniana and Cyathea australis ) and one gymnosperm ( Ginkgo biloba ) species were grown for 2 months in replicated walk-in Conviron BDW40 chambers running at TJB treatment conditions of 16 % [O 2 ]-1900 ppm [CO 2 ] and ambient conditions of 21 % [O 2 ]-400 ppm [CO 2 ], and their physiological plasticity was assessed using gas exchange and chlorophyll fluorescence methods.
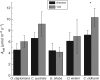
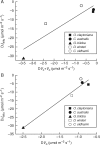
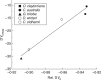
Key results: TJB acclimation caused significant reductions in the maximum rate of carboxylation ( V Cmax ) and the maximum electron flow supporting ribulose-1,5-bisphosphate regeneration ( J max ) in all species, yet this downregulation had little effect on their light-saturated photosynthetic rate ( A sat ). Ginkgo was found to photorespire heavily under ambient conditions, while growth in low [O 2 ]:[CO 2 ] resulted in increased heat dissipation per reaction centre ( DI o / RC ), severe photodamage, as revealed by the species' decreased maximum efficiency of primary photochemistry ( F v / F m ) and decreased in situ photosynthetic electron flow ( Jsitu ).
Conclusions: It is argued that the observed photodamage reflects the inability of Ginkgo to divert excess photosynthetic electron flow to sinks other than the downregulated C 3 and the diminished C 2 cycles under low [O 2 ]:[CO 2 ]. This finding, coupled with the remarkable physiological plasticity of the ferns, provides insights into the underlying mechanism of Ginkgoales' near extinction and ferns' proliferation as atmospheric [CO 2 ] increased to maximum levels across the TJB.
Keywords: Ginkgo biloba; Triassic–Jurassic boundary; angiosperms; gymnosperms; high CO2; low O2; mesophyll conductance; monilophytes; photodamage; photorespiration; photosynthetic plasticity; stomatal conductance.
© The Author 2017. Published by Oxford University Press on behalf of the Annals of Botany Company.
Figures




Similar articles
-
Modelling (18)O2 and (16)O2 unidirectional fluxes in plants. III: fitting of experimental data by a simple model.Biosystems. 2013 Aug;113(2):104-14. doi: 10.1016/j.biosystems.2012.10.004. Epub 2012 Nov 13. Biosystems. 2013. PMID: 23153764
-
The evolution of diffusive and biochemical capacities for photosynthesis was predominantly shaped by [CO2] with a smaller contribution from [O2].Sci Total Environ. 2022 Sep 20;840:156606. doi: 10.1016/j.scitotenv.2022.156606. Epub 2022 Jun 9. Sci Total Environ. 2022. PMID: 35691351
-
Stomatal and mesophyll conductances to CO₂ in different plant groups: underrated factors for predicting leaf photosynthesis responses to climate change?Plant Sci. 2014 Sep;226:41-8. doi: 10.1016/j.plantsci.2014.06.011. Epub 2014 Jun 20. Plant Sci. 2014. PMID: 25113449
-
Role of mesophyll diffusion conductance in constraining potential photosynthetic productivity in the field.J Exp Bot. 2009;60(8):2249-70. doi: 10.1093/jxb/erp036. Epub 2009 Apr 23. J Exp Bot. 2009. PMID: 19395391 Review.
-
Photosynthesis Optimized across Land Plant Phylogeny.Trends Plant Sci. 2019 Oct;24(10):947-958. doi: 10.1016/j.tplants.2019.07.002. Epub 2019 Jul 27. Trends Plant Sci. 2019. PMID: 31362860 Review.
Cited by
-
Phenotypic variation from waterlogging in multiple perennial ryegrass varieties under climate change conditions.Front Plant Sci. 2022 Aug 4;13:954478. doi: 10.3389/fpls.2022.954478. eCollection 2022. Front Plant Sci. 2022. PMID: 35991411 Free PMC article.
-
Plant responses to decadal scale increments in atmospheric CO2 concentration: comparing two stomatal conductance sampling methods.Planta. 2020 Jan 16;251(2):52. doi: 10.1007/s00425-020-03343-z. Planta. 2020. PMID: 31950281 Free PMC article.
-
Minimum levels of atmospheric oxygen from fossil tree roots imply new plant-oxygen feedback.Geobiology. 2021 May;19(3):250-260. doi: 10.1111/gbi.12435. Epub 2021 Feb 19. Geobiology. 2021. PMID: 33608990 Free PMC article.
-
A Novel Hypothesis for the Role of Photosynthetic Physiology in Shaping Macroevolutionary Patterns.Plant Physiol. 2019 Nov;181(3):1148-1162. doi: 10.1104/pp.19.00749. Epub 2019 Sep 4. Plant Physiol. 2019. PMID: 31484680 Free PMC article.
-
Strong increase of photosynthetic pigments and leaf size in a pruned Ginkgo biloba tree.Photosynthetica. 2023 Jun 1;61(3):297-307. doi: 10.32615/ps.2023.020. eCollection 2023. Photosynthetica. 2023. PMID: 39651366 Free PMC article.
References
-
- Ainsworth EA, Long SP.. 2005. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytologist 165: 351–372. - PubMed
-
- Ainsworth EA, Rogers A.. 2007. The response of photosynthesis and stomatal conductance to rising [CO2]: mechanisms and environmental interactions. Plant, Cell & Environment 30: 258–270. - PubMed
-
- Andersson I. 2008. Catalysis and regulation in Rubisco. Journal of Experimental Botany 59: 1555–1568. - PubMed
-
- Ash S. 1986. Fossil plants and the Triassic–Jurassic boundary In: Padian K, ed. The beginning of the age of dinosaurs. Cambridge: Cambridge University Press, 21–30.
-
- Bachan A, van de Schootbrugge B, Feibig J, McRoberts CA, Ciarapica G, Payne JL.. 2012. Carbon cycle dynamics following the end-Triassic mass extinction: constraints from paired d13Ccarb and d13Corg records. Geochemistry, Geophysics, Geosystems 13: 1–24.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

