Effect of KIT and PDGFRA Mutations on Survival in Patients With Gastrointestinal Stromal Tumors Treated With Adjuvant Imatinib: An Exploratory Analysis of a Randomized Clinical Trial
- PMID: 28334365
- PMCID: PMC5470395
- DOI: 10.1001/jamaoncol.2016.5751
Effect of KIT and PDGFRA Mutations on Survival in Patients With Gastrointestinal Stromal Tumors Treated With Adjuvant Imatinib: An Exploratory Analysis of a Randomized Clinical Trial
Abstract
Importance: Little is known about whether the duration of adjuvant imatinib influences the prognostic significance of KIT proto-oncogene receptor tyrosine kinase (KIT) and platelet-derived growth factor receptor α (PDGFRA) mutations.
Objective: To investigate the effect of KIT and PDGFRA mutations on recurrence-free survival (RFS) in patients with gastrointestinal stromal tumors (GISTs) treated with surgery and adjuvant imatinib.
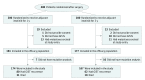
Design, setting, and participants: This exploratory study is based on the Scandinavian Sarcoma Group VIII/Arbeitsgemeinschaft Internistische Onkologie (SSGXVIII/AIO) multicenter clinical trial. Between February 4, 2004, and September 29, 2008, 400 patients who had undergone surgery for GISTs with a high risk of recurrence were randomized to receive adjuvant imatinib for 1 or 3 years. Of the 397 patients who provided consent, 341 (85.9%) had centrally confirmed, localized GISTs with mutation analysis for KIT and PDGFRA performed centrally using conventional sequencing. During a median follow-up of 88 months (completed December 31, 2013), 142 patients had GIST recurrence. Data of the evaluable population were analyzed February 4, 2004, through December 31, 2013.
Main outcomes and measures: The main outcome was RFS. Mutations were grouped by the gene and exon. KIT exon 11 mutations were further grouped as deletion or insertion-deletion mutations, substitution mutations, insertion or duplication mutations, and mutations that involved codons 557 and/or 558.
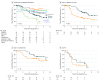
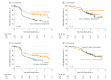
Results: Of the 341 patients (175 men and 166 women; median age at study entry, 62 years) in the 1-year group and 60 years in the 3-year group), 274 (80.4%) had GISTs with a KIT mutation, 43 (12.6%) had GISTs that harbored a PDGFRA mutation, and 24 (7.0%) had GISTs that were wild type for these genes. PDGFRA mutations and KIT exon 11 insertion or duplication mutations were associated with favorable RFS, whereas KIT exon 9 mutations were associated with unfavorable outcome. Patients with KIT exon 11 deletion or insertion-deletion mutation had better RFS when allocated to the 3-year group compared with the 1-year group (5-year RFS, 71.0% vs 41.3%; P < .001), whereas no significant benefit from the 3-year treatment was found in the other mutational subgroups examined. KIT exon 11 deletion mutations, deletions that involved codons 557 and/or 558, and deletions that led to pTrp557_Lys558del were associated with poor RFS in the 1-year group but not in the 3-year group. Similarly, in the subset with KIT exon 11 deletion mutations, higher-than-the-median mitotic counts were associated with unfavorable RFS in the 1-year group but not in the 3-year group.
Conclusions and relevance: Patients with KIT exon 11 deletion mutations benefit most from the longer duration of adjuvant imatinib. The duration of adjuvant imatinib modifies the risk of GIST recurrence associated with some KIT mutations, including deletions that affect exon 11 codons 557 and/or 558.
Trial registration: clinicaltrials.gov Identifier: NCT00116935.
Conflict of interest statement
Figures

Comment in
-
Defining the Impact of Adjuvant Therapy in Molecularly Defined Subsets of Gastrointestinal Stromal Tumor : From Lumping to Splitting.JAMA Oncol. 2017 May 1;3(5):597-599. doi: 10.1001/jamaoncol.2016.5740. JAMA Oncol. 2017. PMID: 28334361 Free PMC article. No abstract available.
-
The big, the bad, and the exon 11: adjuvant imatinib for all gastro-intestinal stromal tumors or just the ugly?Transl Gastroenterol Hepatol. 2017 Oct 13;2:81. doi: 10.21037/tgh.2017.09.10. eCollection 2017. Transl Gastroenterol Hepatol. 2017. PMID: 29167828 Free PMC article. No abstract available.
References
-
- Joensuu H, Hohenberger P, Corless CL. Gastrointestinal stromal tumour. Lancet. 2013;382(9896):973-983. - PubMed
-
- National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology: soft tissue sarcoma. https://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Published 2011. Accessed April 1, 2016.
-
- ESMO/European Sarcoma Network Working Group Gastrointestinal stromal tumours: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(suppl 3):iii21-iii26. - PubMed
-
- Joensuu H, Eriksson M, Sundby Hall K, et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA. 2012;307(12):1265-1272. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

