Dietary interventions for recurrent abdominal pain in childhood
- PMID: 28334433
- PMCID: PMC6464236
- DOI: 10.1002/14651858.CD010972.pub2
Dietary interventions for recurrent abdominal pain in childhood
Abstract
Background: This is an update of the original Cochrane review, last published in 2009 (Huertas-Ceballos 2009). Recurrent abdominal pain (RAP), including children with irritable bowel syndrome, is a common problem affecting between 4% and 25% of school-aged children. For the majority of such children, no organic cause for their pain can be found on physical examination or investigation. Many dietary inventions have been suggested to improve the symptoms of RAP. These may involve either excluding ingredients from the diet or adding supplements such as fibre or probiotics.
Objectives: To examine the effectiveness of dietary interventions in improving pain in children of school age with RAP.
Search methods: We searched CENTRAL, Ovid MEDLINE, Embase, eight other databases, and two trials registers, together with reference checking, citation searching and contact with study authors, in June 2016.
Selection criteria: Randomised controlled trials (RCTs) comparing dietary interventions with placebo or no treatment in children aged five to 18 years with RAP or an abdominal pain-related, functional gastrointestinal disorder, as defined by the Rome III criteria (Rasquin 2006).
Data collection and analysis: We used standard methodological procedures expected by Cochrane. We grouped dietary interventions together by category for analysis. We contacted study authors to ask for missing information and clarification, when needed. We assessed the quality of the evidence for each outcome using the GRADE approach.
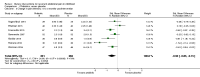
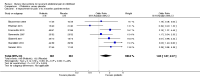
Main results: We included 19 RCTs, reported in 27 papers with a total of 1453 participants. Fifteen of these studies were not included in the previous review. All 19 RCTs had follow-up ranging from one to five months. Participants were aged between four and 18 years from eight different countries and were recruited largely from paediatric gastroenterology clinics. The mean age at recruitment ranged from 6.3 years to 13.1 years. Girls outnumbered boys in most trials. Fourteen trials recruited children with a diagnosis under the broad umbrella of RAP or functional gastrointestinal disorders; five trials specifically recruited only children with irritable bowel syndrome. The studies fell into four categories: trials of probiotic-based interventions (13 studies), trials of fibre-based interventions (four studies), trials of low FODMAP (fermentable oligosaccharides, disaccharides, monosaccharides and polyols) diets (one study), and trials of fructose-restricted diets (one study).We found that children treated with probiotics reported a greater reduction in pain frequency at zero to three months postintervention than those given placebo (standardised mean difference (SMD) -0.55, 95% confidence interval (CI) -0.98 to -0.12; 6 trials; 523 children). There was also a decrease in pain intensity in the intervention group at the same time point (SMD -0.50, 95% CI -0.85 to -0.15; 7 studies; 575 children). However, we judged the evidence for these outcomes to be of low quality using GRADE due to an unclear risk of bias from incomplete outcome data and significant heterogeneity.We found that children treated with probiotics were more likely to experience improvement in pain at zero to three months postintervention than those given placebo (odds ratio (OR) 1.63, 95% CI 1.07 to 2.47; 7 studies; 722 children). The estimated number needed to treat for an additional beneficial outcome (NNTB) was eight, meaning that eight children would need to receive probiotics for one to experience improvement in pain in this timescale. We judged the evidence for this outcome to be of moderate quality due to significant heterogeneity.Children with a symptom profile defined as irritable bowel syndrome treated with probiotics were more likely to experience improvement in pain at zero to three months postintervention than those given placebo (OR 3.01, 95% CI 1.77 to 5.13; 4 studies; 344 children). Children treated with probiotics were more likely to experience improvement in pain at three to six months postintervention compared to those receiving placebo (OR 1.94, 95% CI 1.10 to 3.43; 2 studies; 224 children). We judged the evidence for these two outcomes to be of moderate quality due to small numbers of participants included in the studies.We found that children treated with fibre-based interventions were not more likely to experience an improvement in pain at zero to three months postintervention than children given placebo (OR 1.83, 95% CI 0.92 to 3.65; 2 studies; 136 children). There was also no reduction in pain intensity compared to placebo at the same time point (SMD -1.24, 95% CI -3.41 to 0.94; 2 studies; 135 children). We judged the evidence for these outcomes to be of low quality due to an unclear risk of bias, imprecision, and significant heterogeneity.We found only one study of low FODMAP diets and only one trial of fructose-restricted diets, meaning no pooled analyses were possible.We were unable to perform any meta-analyses for the secondary outcomes of school performance, social or psychological functioning, or quality of daily life, as not enough studies included these outcomes or used comparable measures to assess them.With the exception of one study, all studies reported monitoring children for adverse events; no major adverse events were reported.
Authors' conclusions: Overall, we found moderate- to low-quality evidence suggesting that probiotics may be effective in improving pain in children with RAP. Clinicians may therefore consider probiotic interventions as part of a holistic management strategy. However, further trials are needed to examine longer-term outcomes and to improve confidence in estimating the size of the effect, as well as to determine the optimal strain and dosage. Future research should also explore the effectiveness of probiotics in children with different symptom profiles, such as those with irritable bowel syndrome.We found only a small number of trials of fibre-based interventions, with overall low-quality evidence for the outcomes. There was therefore no convincing evidence that fibre-based interventions improve pain in children with RAP. Further high-quality RCTs of fibre supplements involving larger numbers of participants are required. Future trials of low FODMAP diets and other dietary interventions are also required to facilitate evidence-based recommendations.
Conflict of interest statement
The work of the evidence synthesis team is funded by the National Institute for Health Research Collaboration for Leadership in Applied Health Research and Care South West Peninsula (PenCLAHRC). However, the Funder had no role in the review itself.
Tamsin V Newlove‐Delgado: none known. Alice E Martin: none known. Rebecca A Abbott: none known. Alison Bethel: none known. Joanna Thompson‐Coon: none known. Rebecca Whear: none known Stuart Logan: none known.
The authors who practice clinical paediatrics are Alice E Martin and Stuart Logan. Alice is a Paediatric Trainee and works under the guidance of various Consultant Paediatricians. Stuart is a Consultant Paediatrician and treats children according to current best evidence, in light of their preference. There are therefore no conflicts of interest with this review.
Figures
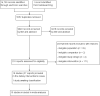
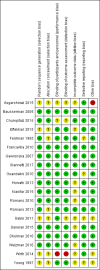
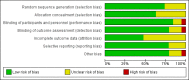

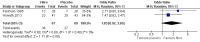








Update of
- doi: 10.1002/14651858.CD010972
References
References to studies included in this review
Asgarshirazi 2015 {published data only}
-
- Asgarshirazi M. Study information [personal communication]. Email to: A Martin 1 November 2016.
-
- Asgarshirazi M, Shariat M, Dalili H. Comparison of the effects of pH‐dependent peppermint oil and synbiotic lactol (Bacillus coagulans + Fructooligosaccharides) on childhood functional abdominal pain: a randomized placebo‐controlled study. Iranian Red Crescent Medical Journal 2015;17(4):e23844. [DOI: 10.5812/ircmj.17(4)2015.23844; PMC4443394; PUBMED: 26023339] - DOI - PMC - PubMed
Bausserman 2005 {published data only}
Chumpitazi 2015 {published data only}
-
- Chumpitazi BP, Cope JL, Hollister EB, Tsai CM, McMeans AR, Luna RA, et al. Randomised clinical trial: gut microbiome biomarkers are associated with clinical response to a low FODMAP diet in children with the irritable bowel syndrome. Alimentary Pharmacology & Therapeutics 2015;42:418‐27. [DOI: 10.1111/apt.13286; PMC4514898; PUBMED: 26104013] - DOI - PMC - PubMed
Eftekhari 2015 {published data only}
Feldman 1985 {published data only}
-
- Feldman W, McGrath P, Hodgson C, Ritter H, Shipman RT. The use of dietary fiber in the management of simple, childhood, idiopathic, recurrent, abdominal pain. Results in a prospective, double‐blind, randomized, controlled trial. American Journal of Diseases of Children 1985;139(12):1216‐8. [PUBMED: 2998181] - PubMed
Francavilla 2010 {published data only}
-
- Francavilla R, Magistà AM, Lionetti E, Canio A, Castellaneta S, Cavallo L. Lactobacillus GG in children with chronic abdominal pain: a double‐blind placebo‐controlled control trial. Digestive and Liver Disease 2009;41 Suppl 3:s217. [DOI: 10.1016/S1590-8658(09)60493-X] - DOI
-
- Francavilla R, Miniello V, Magistà AM, Canio A, Bucci N, Gagliardi F, et al. A randomised controlled trial of Lactobacillus GG in children with functional abdominal pain. Pediatrics 2010;126(6):1445‐51. [PUBMED: 21078735] - PubMed
Gawronska 2007 {published data only}
Giannetti 2017 {published data only (unpublished sought but not used)}
-
- Giannetti E, Alessandrella A, Giovanni D, Campanozzi A, Staiano A, Miele E. A multicenter, randomized, double‐blind, placebo controlled, crossover trial on the efficacy of a mixture of three bifidobacteria in children with functional abdominal pain. Digestive and Liver Disease 2014;46(Suppl 3):e40. [DOI: 10.1016/j.dld.2014.07.042] - DOI
-
- Giannetti E, Maglione M, Alessandrella A, Strisciuglio C, Giovanni D, Campanozzi A, et al. A mixture of 3 bifidobacteria decreases abdominal pain and improves the quality of life in children with irritable bowel syndrome: a multicenter, randomized, double‐blind, placebo‐controlled, crossover trial. Journal of Clinical Gastroenterology 2017;51(1):e5‐10. [DOI: 10.1097/MCG.0000000000000528; PUBMED: 27306945] - DOI - PubMed
-
- Newlove‐Delgado T. Request for study information [personal communication]. Email to: Dr. Staiano 14 November 2016.
Guandalini 2010 {published data only}
-
- Guandalini S, Magazzù G, Chiaro A, Balestra V, Nardo G, Gopalan S, et al. VSL#3 improves symptoms in children with irritable bowel syndrome: a multicenter, randomized, placebo‐controlled, double‐blind, crossover study. Journal of Pediatric Gastroenterology and Nutrition 2010;51(1):24‐30. [DOI: 10.1097/MPG.0b013e3181ca4d95; PUBMED: 20453678] - DOI - PubMed
Horvath 2013 {published data only}
Kianifar 2015 {published data only}
Romano 2010 {published and unpublished data}
-
- Romano C. Systematic review [personal communication]. Email to: Dr R Abbott (RAP review team, University of Exeter, UK) 25 April 2014.
Romano 2013 {published data only}
Sabbi 2011 {published data only}
-
- Sabbi T. The use of lactobacillus GG in children with functional abdominal pain: a double‐blind randomized control trial. Clinical Nutrition Supplements 2011;6(1):198. [DOI: 10.1016/S1744-1161(11)70513-0] - DOI
-
- Sabbi T. The use of lactobacillus GG in children with functional abdominal pain: a double‐blind randomized control trial. Digestive and Liver Disease 2012;44(Suppl 2):S207‐8. [DOI: 10.1016/S1590-8658(12)60589-1] - DOI
-
- Sabbi T, Palumbo M. The use of lactobacillus GG in children with functional abdominal pain: a double‐blind randomized control trial. Digestive and Liver Disease 2011;43(Suppl 5):S412. [DOI: 10.1016/S1590-8658(11)60644-0] - DOI
-
- Whear R. Request for data for Cochrane review [personal communication]. Email to: Dr Sabbi 24 April 2014.
Saneian 2015 {published data only}
Shulman 2016 {published and unpublished data}
-
- Shulman R. Request for raw data for Cochrane systematic review [personal communication]. Email to: T Newlove‐Delgado 22 August 2016.
-
- Shulman RJ, Hollister EB, Cain K, Czyzewski DI, Self MM, Weidler EM, et al. Psyllium fiber reduces abdominal pain in children with Irritable Bowel Syndrome in a randomized, double‐blind trial. Clinical Gastroenterology and Hepatology 2016;9:9. [DOI: 10.1016/j.cgh.2016.03.045; PMC5064811; PUBMED: 27080737] - DOI - PMC - PubMed
Weizman 2016 {published data only}
Wirth 2014 {published data only}
-
- Wirth S, Klodt C, Wintermeyer P, Berrang J, Hensel K, Langer T, et al. Positive or negative fructose breath test results do not predict response to fructose restricted diet in children with recurrent abdominal pain: Results from a prospective randomized trial. Klinische Padiatrie 2014;226(5):268‐73. [DOI: 10.1055/s-0034-1383653; PUBMED: 25153911] - DOI - PubMed
Young 1997 {published data only}
-
- Young RJ. Successful probiotic therapy of chronic recurrent abdominal pain in children. AGA Abstracts Gastroenterology 1997;112(4):A856.
-
- Young RJ, Hanner TL, Vanderhoof JA. Therapeutic efficacy of lactobacillus plantarum 299V in chronic recurrent abdominal pain of childhood. Journal of Pediatric Gastroenterology and Nutrition 1997;25(4):465.
References to studies excluded from this review
Agah 2015 {published data only}
-
- Agah S, Taleb A, Moeini R, Gorji N, Nikbakht H, Soltani‐Kermanshahi M. Chamomile efficacy in patients of the irritable bowel syndrome. Der Pharma Chemica 2015;7(4):237‐41.
Christensen 1982 {published data only}
-
- Christensen MF. Do bulk preparations help in cases of recurrent abdominal pain in children? A controlled study [Hjaelper fyld praeparater pa recidiverende mavesmerter hos born? En kontrolleret study]. Ugeskrift for Laeger 1982;144(10):714‐5. [PUBMED: 7048679] - PubMed
Chumpitazi 2014 {published data only}
-
- Chumpitazi BP, Tsai CM, McMeans AR, Shulman RJ. A low FODMAPS diet ameliorates symptoms in children with irritable bowel syndrome: a double blind, randomized crossover trial. Gastroenterology 2014;146(5 Suppl 1):S‐144. [DOI: 10.1016/S0016-5085(14)60511-4] - DOI
Dearlove 1983 {published data only}
Edwards 1991 {published data only}
-
- Edwards MC, Finney JW, Bonner M. Matching treatment with recurrent abdominal pain symptoms: an evaluation of dietary fiber and relaxation treatments. Behavior Therapy 1991;22(2):257‐67. [DOI: 10.1016/S0005-7894(05)80181-9] - DOI
Lebenthal 1981 {published data only}
-
- Lebenthal E, Rossi TM, Nord KS, Branski D. Recurrrent abdominal pain and lactose absorption in children. Pediatrics 1981;67(6):828‐32. [PUBMED: 7195004] - PubMed
References to studies awaiting assessment
Jarocka‐Cyrta 2002 {published data only}
-
- Jarocka‐Cyrta E, Uscinowicz M, Wasilewska J, Kaczmarski M. The role of cow's milk allergy in recurrent abdominal pain in children above 6 years of age. Double‐blind placebo controlled food challenge. Journal of Pediatric Gastroenterology and Nutrition 2002;34(4):458.
Additional references
Abbott 2017
Abu‐Arafeh 1995
Apley 1958
Asgarshirazi 2016 [pers comm]
-
- Asgarshirazi M. Study information [personal communication]. Email to: A Martin 1 November 2016.
Bain 1974
-
- Bain H. Chronic vague abdominal pain in children. Pediatric Clinics of North America 1974;21(4):991‐1000. [PUBMED: 4427792] - PubMed
Balshem 2011
Bayless 1971
-
- Bayless MD, Huang SS. Recurrent abdominal pain due to milk and lactose intolerance in school‐aged children. Pediatrics 1971;47(6):1029‐32. [PUBMED: 5172188] - PubMed
Bieri 1990
-
- Bieri D, Reeve R, Champion GD, Addicoat L, Ziegler J. The Faces Pain Scale for the self‐assessment of the severity of pain experienced by children: development, initial validation and preliminary investigation for ratio scale properties. Pain 1990;41:139‐50. [PUBMED: 2367140] - PubMed
Claar 2006
Deeks 2011
-
- Deeks JJ, Higgins JPT. Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Devanarayana 2011
-
- Devanarayana NM, Mettananda S, Liyanarachchi C, Nanayakkara N, Mendis N, Perera N, et al. Abdominal pain‐predominant functional gastrointestinal diseases in children and adolescents: prevalence, symptomatology, and association with emotional stress. Journal of Pediatric Gastroenterology and Nutrition 2011;53(6):659‐65. [DOI: 10.1097/MPG.0b013e3182296033; PUBMED: 21697745] - DOI - PubMed
Drossman 1995
-
- Drossman DA, Creed FH, Fava GA, Olden KW, Patrick DL, Toner BB, et al. Psychosocial aspects of the functional gastrointestinal disorders. Gastroenterology International 1995;8(2):47‐90.
Drossman 2016
-
- Drossman DA, Chang L, Chey WD, Kellow J, Tack J, Whitehead WE. Rome IV Functional Gastrointestinal Disorders: Disorders of Gut‐Brain Interaction. Raleigh (NC): Rome Foundation, 2016.
Eccleston 2014
-
- Eccleston C, Palermo T, Williams AC, Lewandowski Holley A, Morley S, Fisher E, et al. Psychological therapies for the management of chronic and recurrent pain in children and adolescents. Cochrane Database of Systematic Reviews 2014, Issue 5. [DOI: 10.1002/14651858.CD003968.pub4] - DOI - PMC - PubMed
Farquar 1956
GRADEpro GDT 2015 [Computer program]
-
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed 12 December 2016. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Guy 1976
-
- Guy W. ECDEU Assessment Manual for Psychopharmacology. Rockville (MD): US Department of Heath, Education, and Welfare Public Health Service Alcohol, Drug Abuse, and Mental Health Administration, 1976.
Harvey 1973
Hicks 2001
-
- Hicks CL, Baeyer CL, Spafford PA, Korlaar I, Goodenough B. The Faces Pain Scale‐Revised: toward a common metric in pediatric pain measurement. Pain 2001;93(2):173‐83. [PUBMED: 11427329] - PubMed
Higgins 2003
Higgins 2011a
-
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
-
- Higgins JPT, Deeks JJ, Altman DG. Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011c
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hockaday 1992
-
- Hockaday JM. Is there a place for "abdominal migraine" as a separate entity in the IHS classification? No!. Cephalalgia 1992;12(6):346‐8. - PubMed
Horst 2014
-
- Horst S, Shelby G, Anderson J, Acra S, Polk DB, Saville BR, et al. Predicting persistence of functional abdominal pain from childhood into young adulthood. Clinical Gastroenterology and Hepatology 2014;12(12):2026‐32. [DOI: 10.1016/j.cgh.2014.03.034; PMC4195814; PUBMED: 24732284] - DOI - PMC - PubMed
Horvath 2011
Horvath 2012
-
- Horvath A, Dziechciarz P, Szajewska H. Systematic review of randomized controlled trials: fiber supplements for abdominal pain‐related functional gastrointestinal disorders in childhood. Annals of Nutrition and Metabolism 2012;61(2):95‐101. [PUBMED: 22889919] - PubMed
Huertas‐Ceballos 2008a
Huertas‐Ceballos 2008b
Hyams 1995
-
- Hyams JS, Treem WR, Justinich CJ, Davis P, Shoup M, Burke G. Characterization of symptoms in children with recurrent abdominal pain: resemblance to irritable bowel syndrome. Journal of Pediatric Gastroenterology and Nutrition 1995;20(2):209‐14. [PUBMED: 7714688] - PubMed
Konijnenberg 2005
Korterink 2015
Lewis 1997
Martin 2014a
McGrath 1996
-
- McGrath PA, Seifert CE, Speechley KN, Booth JC, Stitt L, Gibson MC. A new analogue scale for assessing children's pain: an initial validation study. Pain 1996;64(3):435‐43. [PUBMED: 8783307] - PubMed
Moher 2009
Muller‐Lissner 2003
-
- Müller‐Lissner S, Koch G, Talley NJ, Drossman D, Rueegg P, Dunger‐Baldauf C, et al. Subject's Global Assessment of Relief: an appropriate method to assess the impact of treatment on irritable bowel syndrome‐related symptoms in clinical trials. Journal of Clinical Epidemiology 2003;56(4):310‐6. [PUBMED: 12767407] - PubMed
Nanayakkara 2016
Painter 1964
-
- Painter NS, Truelove SC. Irritable bowel syndrome in childhood. Gut 1964;5:201.
Paul 2013
-
- Paul SP, Candy DC. Clinical update: recurrent abdominal pain in children. Community Practice 2013;86(11):48‐51. [PUBMED: 24369571] - PubMed
Peltonen 1970
-
- Peltonen T. Recurrent abdominal pain. Pediatrics 1970;46(6):973. - PubMed
Quigley 2008
Rao 2015
Rasquin 2006
Review Manager 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collboration, 2014.
Roalfe 2008
Romano 2014 [pers comm]
-
- Romano C. Systematic review [personal communication]. Email to: Dr R Abbott (RAP review team, University of Exeter, UK) 25 April 2014.
Rubin 1967
-
- Rubin LS, Barbero GL, Sibinga MS. Pupillary reactivity in children with recurrent abdominal pain. Psychosomatic Medicine 1967;29(2):111‐20. [PUBMED: 6030151] - PubMed
Rutten 2015
Sandberg 1973
-
- Sandberg DH. Additional references on the tension‐fatigue syndrome. Pediatrics 1973;51(2):308‐9. - PubMed
Scharff 1997
-
- Scharff L. Recurrent abdominal pain in children: a review of psychological factors and treatment. Clinical Psychology Review 1997;17(2):145‐66. [PUBMED: 9140713] - PubMed
Schünemann 2011a
-
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Schünemann 2011b
-
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Shelby 2013
Speer 1954
-
- Speer F. The allergic tension‐fatigue syndrome. Pediatric Clinics of North America 1954;11:1029‐38. [PUBMED: 13204094] - PubMed
Stickler 1979
-
- Stickler GB, Murphy DB. Recurrent abdominal pain. American Journal of Diseases in Children 1979;133(5):486‐9. [PUBMED: 433872] - PubMed
Stone 1970
-
- Stone RT, Barbero GJ. Recurrent abdominal pain in childhood. Pediatrics 1970;45(5):732‐8. - PubMed
Stowens 1970
-
- Stowens D. Recurrent abdominal pain. Pediatrics 1970;46(6):968‐9. - PubMed
Størdal 2005
-
- Størdal K, Nygaard E, Bentsen B. Recurrent abdominal pain: a five‐year follow‐up study. Acta Paediatrica 2005;94(2):234‐6. [PUBMED: 15981760] - PubMed
Sutton 2000
Svedlund 1988
-
- Svedlund J, Sjödin I, Dotevall G. GSRS ‐ a clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Digestive Diseases and Sciences 1988;33(2):129‐34. [PUBMED: 3123181] - PubMed
Symon 1986
-
- Symon DNK, Russell G. Abdominal migraine: a childhood syndrome defined. Cephalalgia 1986;6(4):223‐8. - PubMed
The Grade Working Group 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A (editors). GRADE Handbook: Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. The Grade Working Group, 2013 (update).
Thompson 2000
-
- Thompson WG, Longstreth GL, Drossman DA, Heaton KW, Irvine EJ, Muller‐Lissner SA. Functional bowel disorders. In: Drossman DA, Corazziari E, Talley NJ, Thompson WG, Whitehead WE editor(s). Rome II: The Functional Gastrointestinal Disorders. Diagnosis, Pathophysiology and Treatment. A Multinational Consensus. Lawrence (KS): Allen Press, 2000.
Varni 2015
-
- Varni JW, Bendo CB, Denham J, Shulman RJ, Self MM, Neigut DA, et al. PedsQL Gastrointestinal Symptoms Scales and Gastrointestinal Worry Scales in pediatric patients with functional and organic gastrointestinal diseases in comparison to healthy controls. Quality of Life Research 2015;24(2):363–78. [DOI: 10.1007/s11136-014-0781-x; PUBMED: 25148757] - DOI - PubMed
von Baeyer 2009
Walker 1998
-
- Walker LS, Guite JW, Duke M, Barnard JA, Greene JW. Recurrent abdominal pain: a potential precursor of irritable bowel syndrome in adolescents and young adults. Journal of Pediatrics 1998;132(6):1010‐5. [PUBMED: 9627595] - PubMed
Walker 2006
-
- Walker LS, Caplan A, Rasquin A. Questionnaire on Pediatric Gastrointestinal Symptoms, Rome III Version (QPGS‐RIII). In: Drossman DA, Corazziari E, Delvaux N, Spiller RC, Talley NJ, Thompson WG editor(s). Rome III: The Functional Gastrointestinal Disorders. 3rd Edition. McLean (VA): Degnon Associates, 2006:961‐90.
Wilhelm 2008
-
- Wilhelm SM, Brubaker CM, Varcak EA, Kale‐Pradhan PB. Effectiveness of probiotics in the treatment of irritable bowel syndrome. Pharmacology 2008;28(4):496‐505. - PubMed
Williams 1996
Wong 1988
-
- Wong D, Baker C. Pain in children: comparison of assessment scales. Pediatric Nursing 1988;14(1):9‐17. [PUBMED: 3344163] - PubMed
Youssef 2006
Youssef 2008
References to other published versions of this review
Huertas‐Ceballos 2009
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

