Regulated in Development and DNA Damage Response 1 Deficiency Impairs Autophagy and Mitochondrial Biogenesis in Articular Cartilage and Increases the Severity of Experimental Osteoarthritis
- PMID: 28334504
- PMCID: PMC5489357
- DOI: 10.1002/art.40104
Regulated in Development and DNA Damage Response 1 Deficiency Impairs Autophagy and Mitochondrial Biogenesis in Articular Cartilage and Increases the Severity of Experimental Osteoarthritis
Abstract
Objective: Regulated in development and DNA damage response 1 (REDD1) is an endogenous inhibitor of mechanistic target of rapamycin (mTOR) that regulates cellular stress responses. REDD1 expression is decreased in aged and osteoarthritic (OA) cartilage, and it regulates mTOR signaling and autophagy in articular chondrocytes in vitro. This study was undertaken to investigate the effects of REDD1 deletion in vivo using a mouse model of experimental OA.
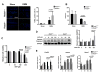
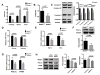
Methods: OA severity was histologically assessed in 4-month-old wild-type and REDD1-/- mice subjected to surgical destabilization of the medial meniscus (DMM). Chondrocyte autophagy, apoptosis, mitochondrial content, and expression of mitochondrial biogenesis markers were determined in cartilage and cultured chondrocytes from wild-type and REDD1-/- mice.
Results: REDD1 deficiency increased the severity of changes in cartilage, menisci, subchondral bone, and synovium in the DMM model of OA. Chondrocyte death was increased in the cartilage of REDD1-/- mice and in cultured REDD1-/- mouse chondrocytes under oxidative stress conditions. Expression of key autophagy markers (microtubule-associated protein 1A/1B light chain 3 and autophagy protein 5) was markedly reduced in cartilage from REDD1-/- mice and in cultured human and mouse chondrocytes with REDD1 depletion. Mitochondrial content, ATP levels, and expression of the mitochondrial biogenesis markers peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α) and transcription factor A, mitochondrial (TFAM) were also decreased in REDD1-deficient chondrocytes. REDD1 was required for AMP-activated protein kinase-induced PGC-1α in chondrocytes.
Conclusion: Our findings suggest that REDD1 is a key mediator of cartilage homeostasis through regulation of autophagy and mitochondrial biogenesis and that REDD1 deficiency exacerbates the severity of injury-induced OA.
© 2017, American College of Rheumatology.
Conflict of interest statement
The authors have no conflicts of interest.
Figures



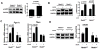
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

