Gradual Suppression of Transcytosis Governs Functional Blood-Retinal Barrier Formation
- PMID: 28334606
- PMCID: PMC5480403
- DOI: 10.1016/j.neuron.2017.02.043
Gradual Suppression of Transcytosis Governs Functional Blood-Retinal Barrier Formation
Abstract
Blood-central nervous system (CNS) barriers partition neural tissues from the blood, providing a homeostatic environment for proper neural function. The endothelial cells that form blood-CNS barriers have specialized tight junctions and low rates of transcytosis to limit the flux of substances between blood and CNS. However, the relative contributions of these properties to CNS barrier permeability are unknown. Here, by studying functional blood-retinal barrier (BRB) formation in mice, we found that immature vessel leakage occurs entirely through transcytosis, as specialized tight junctions are functional as early as vessel entry into the CNS. A functional barrier forms only when transcytosis is gradually suppressed during development. Mutant mice with elevated or reduced levels of transcytosis have delayed or precocious sealing of the BRB, respectively. Therefore, the temporal regulation of transcytosis governs the development of a functional BRB, and suppression of transcytosis is a principal contributor for functional barrier formation.
Keywords: Cav-1; Mfsd2a; blood-CNS barrier; blood-brain barrier; blood-retinal barrier; endothelial cells; pericytes; retinal vasculature; tight junctions; transcytosis.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures
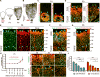

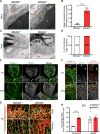

Comment in
-
Filtering more than light in the developing retina.Sci Transl Med. 2017 Apr 19;9(386):eaan2782. doi: 10.1126/scitranslmed.aan2782. Sci Transl Med. 2017. PMID: 28424331
References
-
- Armulik A, Genové G, Mäe M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. - PubMed
-
- Banks WA. From blood-brain barrier to blood-brain interface: new opportunities for CNS drug delivery. Nat Rev Drug Discov 2016 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

