Extended-release naltrexone reduces alcohol consumption among released prisoners with HIV disease as they transition to the community
- PMID: 28334661
- PMCID: PMC5407009
- DOI: 10.1016/j.drugalcdep.2017.01.026
Extended-release naltrexone reduces alcohol consumption among released prisoners with HIV disease as they transition to the community
Abstract
Background: Alcohol use disorders (AUDs) are highly prevalent among persons living with HIV (PLH) within the criminal justice system (CJS). Extended-release naltrexone (XR-NTX) has not been previously evaluated among CJS-involved PLH with AUDs.
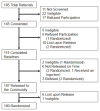
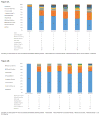
Methods: A randomized, double-blind, placebo-controlled trial was conducted among 100 HIV+ prisoners with AUDs. Participants were randomized 2:1 to receive 6 monthly injections of XR-NTX or placebo starting one week prior to release. Using multiple imputation strategies for data missing completely at random, data were analyzed for the 6-month post-incarceration period. Main outcomes included: time to first heavy drinking day; number of standardized drinks/drinking day; percent of heavy drinking days; pre- to post-incarceration change in average drinks/day; total number of drinking days; and a composite alcohol improvement score comprised of all 5 parameters.
Results: There was no statistically significant difference overall between treatment arms for time-to-heavy-drinking day. However, participants aged 20-29 years who received XR-NTX had a longer time to first heavy drinking day compared to the placebo group (24.1 vs. 9.5days; p<0.001). There were no statistically significant differences between groups for other individual drinking outcomes. A sub-analysis, however, found participants who received ≥4 XR-NTX were more likely (p<0.005) to have improved composite alcohol scores than the placebo group. Post-hoc power analysis revealed that despite the study being powered for HIV outcomes, sufficient power (0.94) was available to distinguish the observed differences.
Conclusions: Among CJS-involved PLH with AUDs transitioning to the community, XR-NTX lengthens the time to heavy drinking day for younger persons; reduces alcohol consumption when using a composite alcohol consumption score; and is not associated with any serious adverse events.
Keywords: Alcohol Use Disorder; Extended-release naltrexone; HIV; Hazardous drinking; Prisoners; Randomized controlled trial.
Published by Elsevier B.V.
Conflict of interest statement
Figures


References
-
- Alkermes Pharmaceuticals. [accessed on January 4, 2010];VIVITROL™ Package Insert. 2006 http://129.128.185.122/drugbank2/drugs/DB00704/fda_labels/153.
-
- Amorim P, Lecrubier Y, Weiller E, Hergueta T, Sheehan D. DSM-IH-R Psychotic Disorders: procedural validity of the Mini International Neuropsychiatric Interview (MINI). Concordance and causes for discordance with the CIDI. Eur Psychiatry. 1998;13:26–34. - PubMed
-
- Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Gastfriend DR, Hosking JD, Johnson BA, LoCastro JS, Longabaugh R, Mason BJ, Mattson ME, Miller WR, Pettinati HM, Randall CL, Swift R, Weiss RD, Williams LD, Zweben A, Group CSR. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: A randomized controlled trial. JAMA. 2006;295:2003–2017. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

