Stabilization of RNA hairpins using non-nucleotide linkers and circularization
- PMID: 28334744
- PMCID: PMC5449636
- DOI: 10.1093/nar/gkx122
Stabilization of RNA hairpins using non-nucleotide linkers and circularization
Abstract
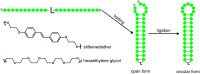
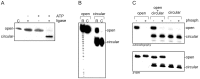
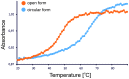
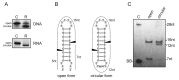
An RNA hairpin is an essential structural element of RNA. Hairpins play crucial roles in gene expression and intermolecular recognition but are also involved in the pathogenesis of some congenital diseases. Structural studies of the hairpin motifs are impeded by their thermodynamic instability, as they tend to unfold to form duplexes, especially at high concentrations required for crystallography or nuclear magnetic resonance spectroscopy. We have elaborated techniques to stabilize the RNA hairpins by linking the free ends of the RNA strand at the base of the hairpin stem. One method involves stilbene diether or hexaethylene glycol linkers and circularization by T4 RNA ligase. Another method uses click chemistry to stitch the RNA ends with a triazole linker. Both techniques are efficient and easy to perform. They should be useful in making stable, biologically relevant RNA constructs for structural studies.
© The Author(s) 2017. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures



References
-
- Bevilacqua P.C., Blose J.M.. Structures, kinetics, thermodynamics, and biological functions of RNA hairpins. Annu.Rev. Phys. Chem. 2008; 59:79–103. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

