Dynamic changes in chromatin states during specification and differentiation of adult intestinal stem cells
- PMID: 28334816
- PMCID: PMC5449638
- DOI: 10.1093/nar/gkx167
Dynamic changes in chromatin states during specification and differentiation of adult intestinal stem cells
Abstract
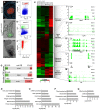
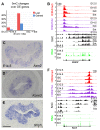
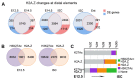
Epigenetic mechanisms, including chromatin structure, chromatin dynamics and histone modifications play an important role for maintenance and differentiation of pluripotent embryonic stem cells. However, little is known about the molecular mechanisms of adult stem cell specification and differentiation. Here, we used intestinal stem cells (ISCs) as a model system to reveal the epigenetic changes coordinating gene expression programs during these processes. We found that two distinct epigenetic mechanisms participate in establishing the transcriptional program promoting ISC specification from embryonic progenitors. A large number of adult ISC signature genes are targets of repressive DNA methylation in embryonic intestinal epithelial progenitors. On the other hand, genes essential for embryonic development acquire H3K27me3 and are silenced during ISC specification. We also show that the repression of ISC signature genes as well as the activation of enterocyte specific genes is accompanied by a global loss of H2A.Z during ISCs differentiation. Our results reveal that, already during ISC specification, an extensive remodeling of chromatin both at promoters and distal regulatory elements organizes transcriptional landscapes operating in differentiated enterocytes, thus explaining similar chromatin modification patterns in the adult gut epithelium.
© The Author(s) 2017. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures




References
-
- Allis C.D., Jenuwein T.. The molecular hallmarks of epigenetic control. Nat. Rev. Genet. 2016; 17:487–500. - PubMed
-
- Lee D.Y., Hayes J.J., Pruss D., Wolffe A.P.. A positive role for histone acetylation in transcription factor access to nucleosomal DNA. Cell. 1993; 72:73–84. - PubMed
-
- Piunti A., Shilatifard A.. Epigenetic balance of gene expression by Polycomb and COMPASS families. Science. 2016; 352:aad9780. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

