Avoidance of APOBEC3B-induced mutation by error-free lesion bypass
- PMID: 28334887
- PMCID: PMC5605239
- DOI: 10.1093/nar/gkx169
Avoidance of APOBEC3B-induced mutation by error-free lesion bypass
Abstract
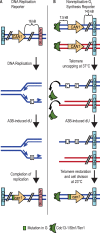
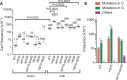
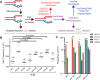
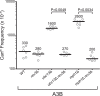
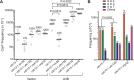
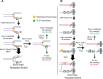
APOBEC cytidine deaminases mutate cancer genomes by converting cytidines into uridines within ssDNA during replication. Although uracil DNA glycosylases limit APOBEC-induced mutation, it is unknown if subsequent base excision repair (BER) steps function on replication-associated ssDNA. Hence, we measured APOBEC3B-induced CAN1 mutation frequencies in yeast deficient in BER endonucleases or DNA damage tolerance proteins. Strains lacking Apn1, Apn2, Ntg1, Ntg2 or Rev3 displayed wild-type frequencies of APOBEC3B-induced canavanine resistance (CanR). However, strains without error-free lesion bypass proteins Ubc13, Mms2 and Mph1 displayed respective 4.9-, 2.8- and 7.8-fold higher frequency of APOBEC3B-induced CanR. These results indicate that mutations resulting from APOBEC activity are avoided by deoxyuridine conversion to abasic sites ahead of nascent lagging strand DNA synthesis and subsequent bypass by error-free template switching. We found this mechanism also functions during telomere re-synthesis, but with a diminished requirement for Ubc13. Interestingly, reduction of G to C substitutions in Ubc13-deficient strains uncovered a previously unknown role of Ubc13 in controlling the activity of the translesion synthesis polymerase, Rev1. Our results highlight a novel mechanism for error-free bypass of deoxyuridines generated within ssDNA and suggest that the APOBEC mutation signature observed in cancer genomes may under-represent the genomic damage these enzymes induce.
© The Author(s) 2017. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures
Similar articles
-
Abasic sites linked to dUTP incorporation in DNA are a major cause of spontaneous mutations in absence of base excision repair and Rad17-Mec3-Ddc1 (9-1-1) DNA damage checkpoint clamp in Saccharomyces cerevisiae.DNA Repair (Amst). 2012 Mar 1;11(3):294-303. doi: 10.1016/j.dnarep.2011.12.004. Epub 2012 Jan 4. DNA Repair (Amst). 2012. PMID: 22226374
-
The choice of nucleotide inserted opposite abasic sites formed within chromosomal DNA reveals the polymerase activities participating in translesion DNA synthesis.DNA Repair (Amst). 2013 Nov;12(11):878-89. doi: 10.1016/j.dnarep.2013.07.008. Epub 2013 Aug 26. DNA Repair (Amst). 2013. PMID: 23988736 Free PMC article.
-
APOBEC3B cytidine deaminase targets the non-transcribed strand of tRNA genes in yeast.DNA Repair (Amst). 2017 May;53:4-14. doi: 10.1016/j.dnarep.2017.03.003. Epub 2017 Mar 21. DNA Repair (Amst). 2017. PMID: 28351647 Free PMC article.
-
Error-free versus mutagenic processing of genomic uracil--relevance to cancer.DNA Repair (Amst). 2014 Jul;19:38-47. doi: 10.1016/j.dnarep.2014.03.028. Epub 2014 Apr 18. DNA Repair (Amst). 2014. PMID: 24746924 Review.
-
Error-free DNA-damage tolerance in Saccharomyces cerevisiae.Mutat Res Rev Mutat Res. 2015 Apr-Jun;764:43-50. doi: 10.1016/j.mrrev.2015.02.001. Epub 2015 Feb 16. Mutat Res Rev Mutat Res. 2015. PMID: 26041265 Review.
Cited by
-
Mesoscale DNA features impact APOBEC3A and APOBEC3B deaminase activity and shape tumor mutational landscapes.Nat Commun. 2024 Mar 18;15(1):2370. doi: 10.1038/s41467-024-45909-5. Nat Commun. 2024. PMID: 38499542 Free PMC article.
-
The Shu complex prevents mutagenesis and cytotoxicity of single-strand specific alkylation lesions.Elife. 2021 Nov 1;10:e68080. doi: 10.7554/eLife.68080. Elife. 2021. PMID: 34723799 Free PMC article.
-
Functional Genomics in Pancreatic β Cells: Recent Advances in Gene Deletion and Genome Editing Technologies for Diabetes Research.Front Endocrinol (Lausanne). 2020 Oct 8;11:576632. doi: 10.3389/fendo.2020.576632. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 33162936 Free PMC article. Review.
-
R-loop formation by dCas9 is mutagenic in Saccharomyces cerevisiae.Nucleic Acids Res. 2019 Mar 18;47(5):2389-2401. doi: 10.1093/nar/gky1278. Nucleic Acids Res. 2019. PMID: 30590793 Free PMC article.
-
Hypomorphic mutation in the large subunit of replication protein A affects mutagenesis by human APOBEC cytidine deaminases in yeast.bioRxiv [Preprint]. 2024 Jul 4:2024.06.27.601081. doi: 10.1101/2024.06.27.601081. bioRxiv. 2024. Update in: G3 (Bethesda). 2024 Oct 7;14(10):jkae196. doi: 10.1093/g3journal/jkae196. PMID: 38979205 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

