RAD51 interconnects between DNA replication, DNA repair and immunity
- PMID: 28334891
- PMCID: PMC5416901
- DOI: 10.1093/nar/gkx126
RAD51 interconnects between DNA replication, DNA repair and immunity
Abstract
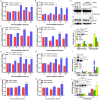
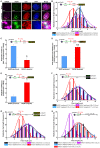
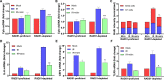
RAD51, a multifunctional protein, plays a central role in DNA replication and homologous recombination repair, and is known to be involved in cancer development. We identified a novel role for RAD51 in innate immune response signaling. Defects in RAD51 lead to the accumulation of self-DNA in the cytoplasm, triggering a STING-mediated innate immune response after replication stress and DNA damage. In the absence of RAD51, the unprotected newly replicated genome is degraded by the exonuclease activity of MRE11, and the fragmented nascent DNA accumulates in the cytosol, initiating an innate immune response. Our data suggest that in addition to playing roles in homologous recombination-mediated DNA double-strand break repair and replication fork processing, RAD51 is also implicated in the suppression of innate immunity. Thus, our study reveals a previously uncharacterized role of RAD51 in initiating immune signaling, placing it at the hub of new interconnections between DNA replication, DNA repair, and immunity.
© The Author(s) 2017. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

