Amelogenesis imperfecta caused by N-terminal enamelin point mutations in mice and men is driven by endoplasmic reticulum stress
- PMID: 28334996
- PMCID: PMC5411757
- DOI: 10.1093/hmg/ddx090
Amelogenesis imperfecta caused by N-terminal enamelin point mutations in mice and men is driven by endoplasmic reticulum stress
Abstract
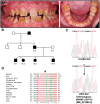
'Amelogenesis imperfecta' (AI) describes a group of inherited diseases of dental enamel that have major clinical impact. Here, we identify the aetiology driving AI in mice carrying a p.S55I mutation in enamelin; one of the most commonly mutated proteins underlying AI in humans. Our data indicate that the mutation inhibits the ameloblast secretory pathway leading to ER stress and an activated unfolded protein response (UPR). Initially, with the support of the UPR acting in pro-survival mode, Enamp.S55I heterozygous mice secreted structurally normal enamel. However, enamel secreted thereafter was structurally abnormal; presumably due to the UPR modulating ameloblast behaviour and function in an attempt to relieve ER stress. Homozygous mutant mice failed to produce enamel. We also identified a novel heterozygous ENAMp.L31R mutation causing AI in humans. We hypothesize that ER stress is the aetiological factor in this case of human AI as it shared the characteristic phenotype described above for the Enamp.S55I mouse. We previously demonstrated that AI in mice carrying the Amelxp.Y64H mutation is a proteinopathy. The current data indicate that AI in Enamp.S55I mice is also a proteinopathy, and based on comparative phenotypic analysis, we suggest that human AI resulting from the ENAMp.L31R mutation is another proteinopathic disease. Identifying a common aetiology for AI resulting from mutations in two different genes opens the way for developing pharmaceutical interventions designed to relieve ER stress or modulate the UPR during enamel development to ameliorate the clinical phenotype.
© The Author 2017. Published by Oxford University Press.
Figures







References
-
- Nanci A. (2007) Ten Cate's Oral Histology: Development, Structure And Function. Mosby, St. Louis, MO.
-
- Bartlett J.D., Ganss B., Goldberg M., Moradian-Oldak J., Paine M.L., Snead M.L., Wen X., White S.N., Zhou Y.L. (2006) 3. Protein-protein interactions of the developing enamel matrix. Curr. Top. Dev. Biol., 74, 57–115. - PubMed
-
- Smith C.E. (1998) Cellular and chemical events during enamel maturation. Crit. Rev. Oral Biol. Med., 9, 128–161. - PubMed
-
- Backman B., Holm A.K. (1986) Amelogenesis imperfecta: prevalence and incidence in a northern Swedish county. Commun. Dent. Oral Epidemiol., 14, 43–47. - PubMed
-
- Coffield K.D., Phillips C., Brady M., Roberts M.W., Strauss R.P., Wright J.T. (2005) The psychosocial impact of developmental dental defects in people with hereditary amelogenesis imperfecta. J. Am. Dent. Assoc., 136, 620–630. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

