Better than sham? A double-blind placebo-controlled neurofeedback study in primary insomnia
- PMID: 28335000
- PMCID: PMC5382955
- DOI: 10.1093/brain/awx011
Better than sham? A double-blind placebo-controlled neurofeedback study in primary insomnia
Abstract
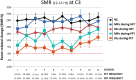
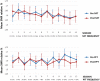
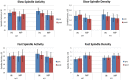
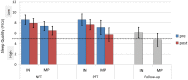
See Thibault et al. (doi:10.1093/awx033) for a scientific commentary on this article.Neurofeedback training builds upon the simple concept of instrumental conditioning, i.e. behaviour that is rewarded is more likely to reoccur, an effect Thorndike referred to as the 'law of effect'. In the case of neurofeedback, information about specific electroencephalographic activity is fed back to the participant who is rewarded whenever the desired electroencephalography pattern is generated. If some kind of hyperarousal needs to be addressed, the neurofeedback community considers sensorimotor rhythm neurofeedback as the gold standard. Earlier treatment approaches using sensorimotor-rhythm neurofeedback indicated that training to increase 12-15 Hz sensorimotor rhythm over the sensorimotor cortex during wakefulness could reduce attention-deficit/hyperactivity disorder and epilepsy symptoms and even improve sleep quality by enhancing sleep spindle activity (lying in the same frequency range). In the present study we sought to critically test whether earlier findings on the positive effect of sensorimotor rhythm neurofeedback on sleep quality and memory could also be replicated in a double-blind placebo-controlled study on 25 patients with insomnia. Patients spent nine polysomnography nights and 12 sessions of neurofeedback and 12 sessions of placebo-feedback training (sham) in our laboratory. Crucially, we found both neurofeedback and placebo feedback to be equally effective as reflected in subjective measures of sleep complaints suggesting that the observed improvements were due to unspecific factors such as experiencing trust and receiving care and empathy from experimenters. In addition, these improvements were not reflected in objective electroencephalographic-derived measures of sleep quality. Furthermore, objective electroencephalographic measures that potentially reflected mechanisms underlying the efficacy of neurofeedback such as spectral electroencephalographic measures and sleep spindle parameters remained unchanged following 12 training sessions. A stratification into 'true' insomnia patients and 'insomnia misperceivers' (subjective, but no objective sleep problems) did not alter the results. Based on this comprehensive and well-controlled study, we conclude that for the treatment of primary insomnia, neurofeedback does not have a specific efficacy beyond unspecific placebo effects. Importantly, we do not find an advantage of neurofeedback over placebo feedback, therefore it cannot be recommended as an alternative to cognitive behavioural therapy for insomnia, the current (non-pharmacological) standard-of-care treatment. In addition, our study may foster a critical discussion that generally questions the effectiveness of neurofeedback, and emphasizes the importance of demonstrating neurofeedback efficacy in other study samples and disorders using truly placebo and double-blind controlled trials.
Keywords: SMR; insomnia; neurofeedback; neurotherapy; placebo.
© The Author (2017). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures

Comment in
-
Neurofeedback or neuroplacebo?Brain. 2017 Apr 1;140(4):862-864. doi: 10.1093/brain/awx033. Brain. 2017. PMID: 28375458 No abstract available.
-
On assessing neurofeedback effects: should double-blind replace neurophysiological mechanisms?Brain. 2017 Oct 1;140(10):e63. doi: 10.1093/brain/awx211. Brain. 2017. PMID: 28969378 No abstract available.
-
Reply: On assessing neurofeedback effects: should double-blind replace neurophysiological mechanisms?Brain. 2017 Oct 1;140(10):e64. doi: 10.1093/brain/awx212. Brain. 2017. PMID: 28969379 Free PMC article. No abstract available.
References
-
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders, DSM-IV. 4th edn. Washington, DC: American Psychiatric Association; 1995.
-
- Anderer P, Gruber G, Parapatics S, Woertz M, Miazhynskaia T, Klosch G. et al. An E-health solution for automatic sleep classification according to Rechtschaffen and Kales: validation study of the Somnolyzer 24 x 7 utilizing the Siesta database. Neuropsychobiology 2005; 51: 115–33. - PubMed
-
- Anderer P, Klosch G, Gruber G, Trenker E, Pascual-Marqui RD, Zeitlhofer J. et al. Low-resolution brain electromagnetic tomography revealed simultaneously active frontal and parietal sleep spindle sources in the human cortex. Neuroscience 2001; 103: 581–92. - PubMed
-
- Arns M, de Ridder S, Strehl U, Breteler M, Coenen A. Efficacy of neurofeedback treatment in ADHD: the effects on inattention, impulsivity and hyperactivity: a meta-analysis. Clin EEG Neurosci 2009; 40: 180–9. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

