Rapamycin Loaded Solid Lipid Nanoparticles as a New Tool to Deliver mTOR Inhibitors: Formulation and in Vitro Characterization
- PMID: 28335215
- PMCID: PMC5302501
- DOI: 10.3390/nano6050087
Rapamycin Loaded Solid Lipid Nanoparticles as a New Tool to Deliver mTOR Inhibitors: Formulation and in Vitro Characterization
Abstract
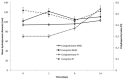
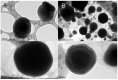
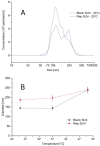
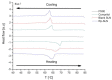
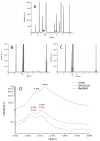
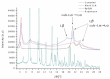
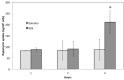
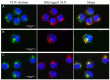
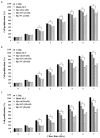
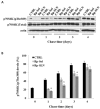
Recently, the use of mammalian target of rapamycin (mTOR) inhibitors, in particular rapamycin (Rp), has been suggested to improve the treatment of neurodegenerative diseases. However, as Rp is a strong immunosuppressant, specific delivery to the brain has been postulated to avoid systemic exposure. In this work, we fabricated new Rp loaded solid lipid nanoparticles (Rp-SLN) stabilized with polysorbate 80 (PS80), comparing two different methods and lipids. The formulations were characterized by differential scanning calorimetry (DSC), nuclear magnetic resonance (NMR), wide angle X-ray scattering (WAXS), cryo-transmission electron microscopy (cryo-TEM), dynamic light scattering (DLS) and particle tracking. In vitro release and short-term stability were assessed. Biological behavior of Rp-SLN was tested in SH-SY5Y neuroblastoma cells. The inhibition of mTOR complex 1 (mTORC1) was evaluated over time by a pulse-chase study compared to free Rp and Rp nanocrystals. Compritol Rp-SLN resulted more stable and possessing proper size and surface properties with respect to cetyl palmitate Rp-SLN. Rapamycin was entrapped in an amorphous form in the solid lipid matrix that showed partial crystallinity with stable Lβ, sub-Lα and Lβ' arrangements. PS80 was stably anchored on particle surface. No drug release was observed over 24 h and Rp-SLN had a higher cell uptake and a more sustained effect over a week. The mTORC1 inhibition was higher with Rp-SLN. Overall, compritol Rp-SLN show suitable characteristics and stability to be considered for further investigation as Rp brain delivery system.
Keywords: SH-SY5Y neuroblastoma cells; drug delivery; formulation; rapamycin; solid lipid nanoparticles.
Conflict of interest statement
The authors declare no conflicts of interest. The founding sponsor had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

