A Label-Free Microelectrode Array Based on One-Step Synthesis of Chitosan-Multi-Walled Carbon Nanotube-Thionine for Ultrasensitive Detection of Carcinoembryonic Antigen
- PMID: 28335260
- PMCID: PMC5224606
- DOI: 10.3390/nano6070132
A Label-Free Microelectrode Array Based on One-Step Synthesis of Chitosan-Multi-Walled Carbon Nanotube-Thionine for Ultrasensitive Detection of Carcinoembryonic Antigen
Abstract
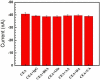
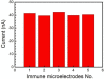
Carcinoembryonic antigen (CEA) has been an extensively used tumor marker responsible for clinical early diagnosis of cervical carcinomas, and pancreatic, colorectal, gastric and lung cancer. Combined with micro-electro mechanical system (MEMS) technology, it is important to develop a novel immune microelectrode array (MEA) not only for rapid analysis of serum samples, but also for cell detection in vitro and in vivo. In this work, we depict a simple approach to modify chitosan-multi-walled carbon nanotubes-thionine (CS-MWCNTs-THI) hybrid film through one-step electrochemical deposition and the CS-MWCNTs-THI hybrid films are successfully employed to immobilize anti-CEA for fabricating simple, label-free, and highly sensitive electro-chemical immune MEAs. The detection principle of immune MEA was based on the fact that the increasing formation of the antigen-antibody immunocomplex resulted in the decreased response currents and the relationship between the current reductions with the corresponding CEA concentrations was directly proportional. Experimental results indicated that the label-free MEA had good selectivity and the limit of detection for CEA is 0.5 pg/mL signal to noise ratio (SNR) = 3. A linear calibration plot for the detection of CEA was obtained in a wide concentration range from 1 pg/mL to 100 ng/mL (r = 0.996). This novel MEA has potential applications for detecting CEA for the research on cancer cells and cancer tissue slices as well as for effective early diagnosis.
Keywords: carcinoembryonic antigen; label-free; microelectrode array; multi-walled carbon nanotube; one-step synthesis.
Figures






Similar articles
-
AuNPs/CNOs/SWCNTs/chitosan-nanocomposite modified electrochemical sensor for the label-free detection of carcinoembryonic antigen.Biosens Bioelectron. 2018 Jun 1;107:211-217. doi: 10.1016/j.bios.2018.02.037. Epub 2018 Feb 14. Biosens Bioelectron. 2018. PMID: 29471282
-
A novel label-free microfluidic paper-based immunosensor for highly sensitive electrochemical detection of carcinoembryonic antigen.Biosens Bioelectron. 2016 Sep 15;83:319-26. doi: 10.1016/j.bios.2016.04.062. Epub 2016 Apr 21. Biosens Bioelectron. 2016. PMID: 27132007
-
The label-free immunosensor based on rhodium@palladium nanodendrites/sulfo group functionalized multi-walled carbon nanotubes for the sensitive analysis of carcino embryonic antigen.Anal Chim Acta. 2018 May 12;1007:61-70. doi: 10.1016/j.aca.2017.12.030. Epub 2017 Dec 30. Anal Chim Acta. 2018. PMID: 29405989
-
Fabrication of an ultrasensitive and selective electrochemical aptasensor to detect carcinoembryonic antigen by using a new nanocomposite.Biosens Bioelectron. 2019 Mar 15;129:1-6. doi: 10.1016/j.bios.2018.12.047. Epub 2019 Jan 3. Biosens Bioelectron. 2019. PMID: 30677696
-
Carbon nanotube-based symbiotic coaxial nanocables with nanosilica and nanogold particles as labels for electrochemical immunoassay of carcinoembryonic antigen in biological fluids.Talanta. 2011 Apr 15;84(2):538-46. doi: 10.1016/j.talanta.2011.01.063. Epub 2011 Feb 1. Talanta. 2011. PMID: 21376985
Cited by
-
Nanoscale dynamic chemical, biological sensor material designs for control monitoring and early detection of advanced diseases.Mater Today Bio. 2020 Feb 14;5:100044. doi: 10.1016/j.mtbio.2020.100044. eCollection 2020 Jan. Mater Today Bio. 2020. PMID: 32181446 Free PMC article. Review.
-
Single-step and ultrasensitive detection of carcinoembryonic antigen based on an aptamer transduction-mediated exonuclease III-assisted dual-amplification strategy.RSC Adv. 2018 Apr 18;8(26):14663-14668. doi: 10.1039/c8ra00416a. eCollection 2018 Apr 17. RSC Adv. 2018. PMID: 35540776 Free PMC article.
-
Electrochemical Immunosensor Using Electroactive Carbon Nanohorns for Signal Amplification for the Rapid Detection of Carcinoembryonic Antigen.Biosensors (Basel). 2022 Dec 30;13(1):63. doi: 10.3390/bios13010063. Biosensors (Basel). 2022. PMID: 36671898 Free PMC article.
-
Carbon Nanomaterial Based Biosensors for Non-Invasive Detection of Cancer and Disease Biomarkers for Clinical Diagnosis.Sensors (Basel). 2017 Aug 20;17(8):1919. doi: 10.3390/s17081919. Sensors (Basel). 2017. PMID: 28825646 Free PMC article. Review.
References
-
- Wang R., Chen X., Ma J., Ma Z. Ultrasensitive detection of carcinoembryonic antigen by a simple label-free immunosensor. Sens. Actuators B. 2013;176:1044–1050. doi: 10.1016/j.snb.2012.10.001. - DOI
-
- Kong F.Y., Zhu X., Xu M.T., Xu J.J., Chen H.Y. Gold nanoparticle/DNA/methylene blue nanocomposites for the ultrasensitive electrochemical detection of carcinoembryonic antigen. Anal. Chim. Acta. 2011;56:9386–9390. doi: 10.1016/j.electacta.2011.08.018. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

