Synthesis of Substituted α-Trifluoromethyl Piperidinic Derivatives
- PMID: 28335499
- PMCID: PMC6155269
- DOI: 10.3390/molecules22030483
Synthesis of Substituted α-Trifluoromethyl Piperidinic Derivatives
Abstract
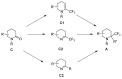
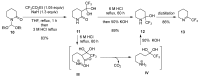
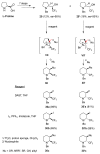
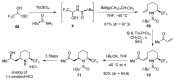
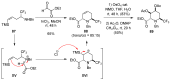
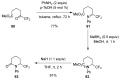
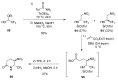
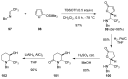
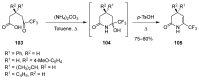
A comprehensive survey of pathways leading to the generation of α-trifluoromethyl monocyclic piperidinic derivatives is provided (65 references). These compounds have been synthesized either from 6-membered rings e.g., pipecolic acid or lactam derivatives by introduction a trifluoromethyl group, from pyridine or pyridinone derivatives by reduction, and from 5-membered rings e.g., prolinol derivatives by ring expansion, from linear amines by cyclization or from dienes/dienophiles by [4 + 2]-cycloaddition.
Keywords: cyclization; cycloaddition; fluorine; nitrogen heterocycles; piperidine; ring expansion; trifluoromethyl group.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

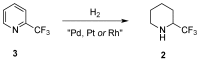
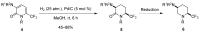







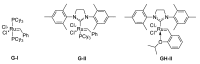




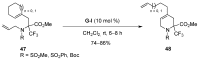


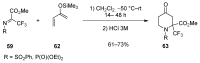








References
-
- Laschat S., Dickner T. Stereoselective synthesis of piperidines. Synthesis. 2000:1781–1813. doi: 10.1055/s-2000-8218. - DOI
-
- Toyooka N., Nemoto H. Application of chiral building blocks to the synthesis of drugs. Drugs Fut. 2002;27:143–158. doi: 10.1358/dof.2002.027.02.654810. - DOI
-
- Felpin F.-X., Lebreton J. Recent advances in the total synthesis of piperidine and pyrrolidine natural alkaloids with ring-closing metathesis as a key step. Eur. J. Org. Chem. 2003:3693–3712. doi: 10.1002/ejoc.200300193. - DOI
-
- Basra S., Blechert S. Ring rearrangement metathesis—A new concept in piperidine and pyrrolidine synthesis. Strategies Tactics Org. Synt. 2004;4:315–346.
-
- Pearson M.S.M., Mathe-Allainmat M., Fargeas V., Lebreton J. Recent advances in the total synthesis of piperidine aza-sugars. Eur. J. Org. Chem. 2005:2159–2191. doi: 10.1002/ejoc.200400823. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

