Synthesis of Randomly Substituted Anionic Cyclodextrins in Ball Milling
- PMID: 28335503
- PMCID: PMC6155213
- DOI: 10.3390/molecules22030485
Synthesis of Randomly Substituted Anionic Cyclodextrins in Ball Milling
Abstract
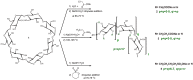
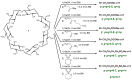
A number of influencing factors mean that the random substitution of cyclodextrins (CD) in solution is difficult to reproduce. Reaction assembly in mechanochemistry reduces the number of these factors. However, lack of water can improve the reaction outcomes by minimizing the reagent's hydrolysis. High-energy ball milling is an efficient, green and simple method for one-step reactions and usually reduces degradation and byproduct formation. Anionic CD derivatives have successfully been synthesized in the solid state, using a planetary ball mill. Comparison with solution reactions, the solvent-free conditions strongly reduced the reagent hydrolysis and resulted in products of higher degree of substitution (DS) with more homogeneous DS distribution. The synthesis of anionic CD derivatives can be effectively performed under mechanochemical activation without significant changes to the substitution pattern but the DS distributions were considerably different from the products of solution syntheses.
Keywords: anionic cyclodextrin; high-energy ball milling; random substitution; solvent-free reactions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Reaction of oxiranes with cyclodextrins under high-energy ball-milling conditions.Beilstein J Org Chem. 2019 Jul 1;15:1448-1459. doi: 10.3762/bjoc.15.145. eCollection 2019. Beilstein J Org Chem. 2019. PMID: 31354861 Free PMC article.
-
Influence of the milling parameters on the nucleophilic substitution reaction of activated β-cyclodextrins.Beilstein J Org Chem. 2017 Sep 7;13:1893-1899. doi: 10.3762/bjoc.13.184. eCollection 2017. Beilstein J Org Chem. 2017. PMID: 29062408 Free PMC article.
-
Toward a Greener World-Cyclodextrin Derivatization by Mechanochemistry.Molecules. 2021 Aug 27;26(17):5193. doi: 10.3390/molecules26175193. Molecules. 2021. PMID: 34500627 Free PMC article. Review.
-
Efficient mechanochemical synthesis of regioselective persubstituted cyclodextrins.Beilstein J Org Chem. 2016 Nov 10;12:2364-2371. doi: 10.3762/bjoc.12.230. eCollection 2016. Beilstein J Org Chem. 2016. PMID: 28144304 Free PMC article.
-
Comparison of the Conventional and Mechanochemical Syntheses of Cyclodextrin Derivatives.Molecules. 2023 Jan 4;28(2):467. doi: 10.3390/molecules28020467. Molecules. 2023. PMID: 36677527 Free PMC article. Review.
Cited by
-
Reaction of oxiranes with cyclodextrins under high-energy ball-milling conditions.Beilstein J Org Chem. 2019 Jul 1;15:1448-1459. doi: 10.3762/bjoc.15.145. eCollection 2019. Beilstein J Org Chem. 2019. PMID: 31354861 Free PMC article.
-
Influence of the milling parameters on the nucleophilic substitution reaction of activated β-cyclodextrins.Beilstein J Org Chem. 2017 Sep 7;13:1893-1899. doi: 10.3762/bjoc.13.184. eCollection 2017. Beilstein J Org Chem. 2017. PMID: 29062408 Free PMC article.
-
Toward a Greener World-Cyclodextrin Derivatization by Mechanochemistry.Molecules. 2021 Aug 27;26(17):5193. doi: 10.3390/molecules26175193. Molecules. 2021. PMID: 34500627 Free PMC article. Review.
-
In Vitro Enhanced Skin Permeation and Retention of Imiquimod Loaded in β-Cyclodextrin Nanosponge Hydrogel.Pharmaceutics. 2019 Mar 20;11(3):138. doi: 10.3390/pharmaceutics11030138. Pharmaceutics. 2019. PMID: 30897794 Free PMC article.
-
Mechanochemical green synthesis of hyper-crosslinked cyclodextrin polymers.Beilstein J Org Chem. 2020 Jun 29;16:1554-1563. doi: 10.3762/bjoc.16.127. eCollection 2020. Beilstein J Org Chem. 2020. PMID: 32704321 Free PMC article.
References
-
- Szejtli J., Osa T. Cyclodextrins. In: Atwood J.L., Lehn J.M., editors. Comprehensive Supramolecular Chemistry. Volume 3. Elsevier; Oxford, UK (aka Pergamon Press): 1996. pp. 1–693.
-
- Jicsinszky L., Hashimoto H., Fenyvesi E., Ueno A. Cyclodextrin derivatives. In: Szejtli J., Osa T., editors. Comprehensive Supramolecular Chemistry. Volume 3. Elsevier; Oxford, UK (aka Pergamon Press): 1996. pp. 57–188.
-
- Jicsinszky L. Regioselective substitution of cyclodextrins without using protecting groups. In: Labandeira J.J.T., Vila-Jato J.L., editors. Proc. Ninth Int. Symp. Cyclodextrins. Kluwer Academic Publishers; Dordrecht, The Netherlands: 1999. pp. 513–516.
-
- Jicsinszky L., Szejtli J. Effect of catalysts on the acylation of cyclodextrins. In: Hedges A.R., editor. Minutes of Sixth Int. Symp. Cyclodextrins. Éditions de Santé; Paris, France: 1992. pp. 96–100.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

