Complete and Incomplete Hepatitis B Virus Particles: Formation, Function, and Application
- PMID: 28335554
- PMCID: PMC5371811
- DOI: 10.3390/v9030056
Complete and Incomplete Hepatitis B Virus Particles: Formation, Function, and Application
Abstract
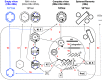
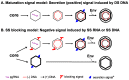
Hepatitis B virus (HBV) is a para-retrovirus or retroid virus that contains a double-stranded DNA genome and replicates this DNA via reverse transcription of a RNA pregenome. Viral reverse transcription takes place within a capsid upon packaging of the RNA and the viral reverse transcriptase. A major characteristic of HBV replication is the selection of capsids containing the double-stranded DNA, but not those containing the RNA or the single-stranded DNA replication intermediate, for envelopment during virion secretion. The complete HBV virion particles thus contain an outer envelope, studded with viral envelope proteins, that encloses the capsid, which, in turn, encapsidates the double-stranded DNA genome. Furthermore, HBV morphogenesis is characterized by the release of subviral particles that are several orders of magnitude more abundant than the complete virions. One class of subviral particles are the classical surface antigen particles (Australian antigen) that contain only the viral envelope proteins, whereas the more recently discovered genome-free (empty) virions contain both the envelope and capsid but no genome. In addition, recent evidence suggests that low levels of RNA-containing particles may be released, after all. We will summarize what is currently known about how the complete and incomplete HBV particles are assembled. We will discuss briefly the functions of the subviral particles, which remain largely unknown. Finally, we will explore the utility of the subviral particles, particularly, the potential of empty virions and putative RNA virions as diagnostic markers and the potential of empty virons as a vaccine candidate.
Keywords: Australian antigen; CCC DNA; HBcAg; HBsAg; diagnosis; empty virion; hepatitis B virus; subviral particles; vaccine; virion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Hu J. Hepatitis B virus virology and replication. In: Liaw Y.-F., Zoulim F., editors. Hepatitis B Virus in Human Diseases. Humana Press; New York, NY, USA: Dordrecht, The Netherlands: London, UK: 2016. pp. 1–34.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

