Testis-specific ATP synthase peripheral stalk subunits required for tissue-specific mitochondrial morphogenesis in Drosophila
- PMID: 28335714
- PMCID: PMC5364652
- DOI: 10.1186/s12860-017-0132-1
Testis-specific ATP synthase peripheral stalk subunits required for tissue-specific mitochondrial morphogenesis in Drosophila
Abstract
Background: In Drosophila early post-meiotic spermatids, mitochondria undergo dramatic shaping into the Nebenkern, a spherical body with complex internal structure that contains two interwrapped giant mitochondrial derivatives. The purpose of this study was to elucidate genetic and molecular mechanisms underlying the shaping of this structure.
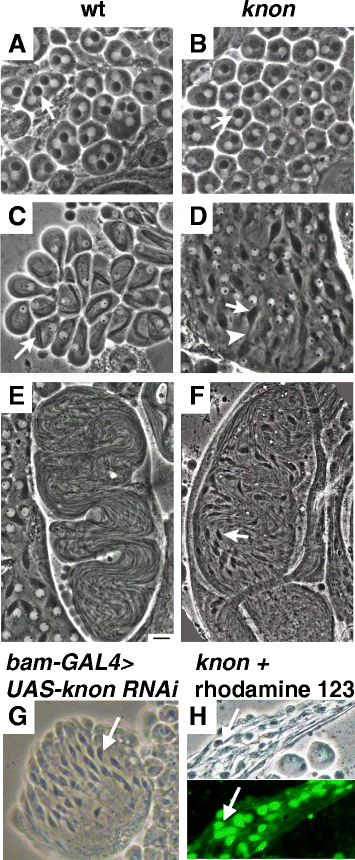
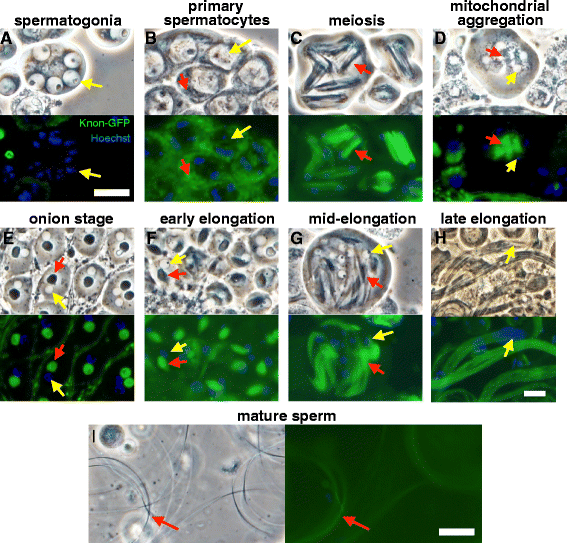
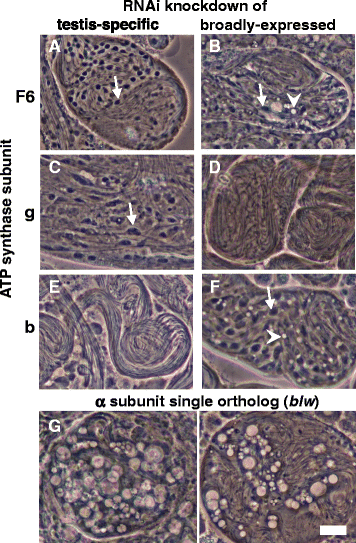
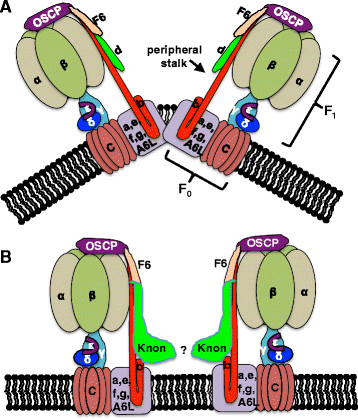
Results: The knotted onions (knon) gene encodes an unconventionally large testis-specific paralog of ATP synthase subunit d and is required for internal structure of the Nebenkern as well as its subsequent disassembly and elongation. Knon localizes to spermatid mitochondria and, when exogenously expressed in flight muscle, alters the ratio of ATP synthase complex dimers to monomers. By RNAi knockdown we uncovered mitochondrial shaping roles for other testis-expressed ATP synthase subunits.
Conclusions: We demonstrate the first known instance of a tissue-specific ATP synthase subunit affecting tissue-specific mitochondrial morphogenesis. Since ATP synthase dimerization is known to affect the degree of inner mitochondrial membrane curvature in other systems, the effect of Knon and other testis-specific paralogs of ATP synthase subunits may be to mediate differential membrane curvature within the Nebenkern.
Keywords: ATP synthase; Cristae; Drosophila melanogaster; Mitochondria; Spermatogenesis.
Figures



Similar articles
-
Spermitin: a novel mitochondrial protein in Drosophila spermatids.PLoS One. 2014 Sep 29;9(9):e108802. doi: 10.1371/journal.pone.0108802. eCollection 2014. PLoS One. 2014. PMID: 25265054 Free PMC article.
-
Testis-Specific Bb8 Is Essential in the Development of Spermatid Mitochondria.PLoS One. 2016 Aug 16;11(8):e0161289. doi: 10.1371/journal.pone.0161289. eCollection 2016. PLoS One. 2016. PMID: 27529784 Free PMC article.
-
Cardiolipin affects the supramolecular organization of ATP synthase in mitochondria.Biophys J. 2011 May 4;100(9):2184-92. doi: 10.1016/j.bpj.2011.03.031. Biophys J. 2011. PMID: 21539786 Free PMC article.
-
The structure and function of mitochondrial F1F0-ATP synthases.Int Rev Cell Mol Biol. 2008;267:1-58. doi: 10.1016/S1937-6448(08)00601-1. Int Rev Cell Mol Biol. 2008. PMID: 18544496 Review.
-
How rotating ATP synthases can modulate membrane structure.Arch Biochem Biophys. 2021 Sep 15;708:108939. doi: 10.1016/j.abb.2021.108939. Epub 2021 May 28. Arch Biochem Biophys. 2021. PMID: 34052190 Review.
Cited by
-
COX4-like, a Nuclear-Encoded Mitochondrial Gene Duplicate, Is Essential for Male Fertility in Drosophila melanogaster.Genes (Basel). 2022 Feb 25;13(3):424. doi: 10.3390/genes13030424. Genes (Basel). 2022. PMID: 35327978 Free PMC article.
-
Silencing of Rieske Iron-Sulfur Protein Impacts Upon the Development and Reproduction of Spodoptera exigua by Regulating ATP Synthesis.Front Physiol. 2018 May 24;9:575. doi: 10.3389/fphys.2018.00575. eCollection 2018. Front Physiol. 2018. PMID: 29881355 Free PMC article.
-
Condensed Mitochondria Assemble Into the Acrosomal Matrix During Spermiogenesis.Front Cell Dev Biol. 2022 Apr 21;10:867175. doi: 10.3389/fcell.2022.867175. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35531097 Free PMC article.
-
Deficiency of ValRS-m Causes Male Infertility in Drosophila melanogaster.Int J Mol Sci. 2024 Jul 8;25(13):7489. doi: 10.3390/ijms25137489. Int J Mol Sci. 2024. PMID: 39000597 Free PMC article.
-
ATP synthase F1 subunits recruited to centromeres by CENP-A are required for male meiosis.Nat Commun. 2018 Jul 13;9(1):2702. doi: 10.1038/s41467-018-05093-9. Nat Commun. 2018. PMID: 30006572 Free PMC article.
References
-
- Tates AD. Cytodifferentiation during spermatogenesis in Drosophila melanogaster: an electron microscope study. Leiden: Rijksuniversiteit; 1971.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

