SVR Rates of HCV-infected population under PEG-IFN-α/R treatment in Northwest China
- PMID: 28335783
- PMCID: PMC5364675
- DOI: 10.1186/s12985-017-0708-6
SVR Rates of HCV-infected population under PEG-IFN-α/R treatment in Northwest China
Abstract
Background: Chronic HCV Patients taking PEG-IFN-α/R from different ethnic groups have different probabilities of reaching a sustained viral response (SVR). There are many influence factors, such as HCV genotype, IL-28B single-nucleotide polymorphisms (SNP), Fibrosis 4 index (FIB-4), and aspartate aminotransferase-to-platelet ratio index (APRI) score. But the baseline factors in relation to treatment outcome was still not much clear.
Methods: We evaluated data from 231 chronic HCV patients with or without liver fibrosis and their antiviral efficacy after treatment with pegylated interferon plus ribavirin (PEG-IFN-α/R) for 24-48 weeks. IL-28B SNP and HCV genotypes were analyzed with genome sequencing using pyrosequencing.
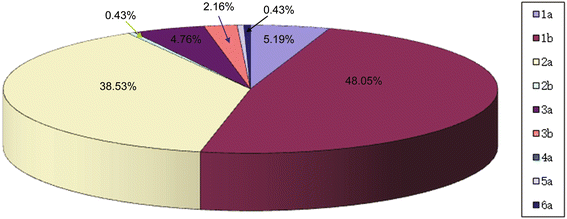
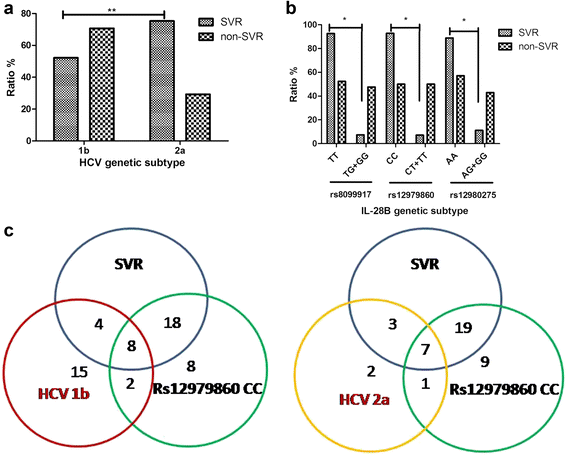
Results: Sustained viral response (SVR) rates of patients with HCV 1b and 2a genotypes were 52.25% (58/111) and 75.28% (67/89) (P < 0.01). SVR rates of patients with IL-28B rs8099917 TT, rs12979860 CC and rs12980275 AA were 92.41% (25/27), 92.86% (26/28) and 88.89% (24/27) separately. We found that SVR rates in HCV 1b and 2a patients were only 31.0 and 39.4% if their FIB-4 > 3.25. In addition, when their APRI > 2, only 30.3% of HCV 1b patients and 50.2% of HCV 2a patients could obtain SVR.
Conclusions: There were high proportion of HCV genotype 1b and 2a in Northwest China. In both HCV 1b and 2a genotypes, patients with protective-genotype of IL-28B were more likely to obtain SVR. However, those with significant fibrosis or cirrhosis were less likely, no matter their genotype. Combined factors of HCV genotype, IL-28B genotype, FIB-4 and ARPI may indicate high prediction and clinical value regarding treatment with PEG-IFN-α/R and prognostic evaluation of chronic hepatitis C patients.
Keywords: Chronic hepatitis C; Cirrhosis; HCV subtype; Individualization; Interleukin 28; Single nucleotide polymorphism.
Figures
References
-
- Antonelli A, Pistello M. New Therapies, Markers and Therapeutic Targets in HCV Chronic Infection, and HCV Extrahepatic Manifestations. Curr Drug Targets. 2015. - PubMed
-
- Pellicelli AM, Romano M, Stroffolini T, Mazzoni E, Mecenate F, Monarca R, Picardi A, Bonaventura ME, Mastropietro C, Vignally P, et al. HCV genotype 1a shows a better virological response to antiviral therapy than HCV genotype 1b. BMC Gastroenterol. 2012;12:162. doi: 10.1186/1471-230X-12-162. - DOI - PMC - PubMed
-
- Lawitz E, Poordad FF, Pang PS, Hyland RH, Ding X, Mo H, Symonds WT, McHutchison JG, Membreno FE. Sofosbuvir and ledipasvir fixed-dose combination with and without ribavirin in treatment-naive and previously treated patients with genotype 1 hepatitis C virus infection (LONESTAR): an open-label, randomised, phase 2 trial. Lancet. 2014;383:515–523. doi: 10.1016/S0140-6736(13)62121-2. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

