NEOSCOPE: A randomised phase II study of induction chemotherapy followed by oxaliplatin/capecitabine or carboplatin/paclitaxel based pre-operative chemoradiation for resectable oesophageal adenocarcinoma
- PMID: 28335886
- PMCID: PMC5341738
- DOI: 10.1016/j.ejca.2016.11.031
NEOSCOPE: A randomised phase II study of induction chemotherapy followed by oxaliplatin/capecitabine or carboplatin/paclitaxel based pre-operative chemoradiation for resectable oesophageal adenocarcinoma
Abstract
Background: Oxaliplatin-capecitabine (OxCap) and carboplatin-paclitaxel (CarPac) based neo-adjuvant chemoradiotherapy (nCRT) have shown promising activity in localised, resectable oesophageal cancer.
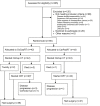
Patients and methods: A non-blinded, randomised (1:1 via a centralised computer system), 'pick a winner' phase II trial. Patients with resectable oesophageal adenocarcinoma ≥ cT3 and/or ≥ cN1 were randomised to OxCapRT (oxaliplatin 85 mg/m2 day 1, 15, 29; capecitabine 625 mg/m2 bd on days of radiotherapy) or CarPacRT (carboplatin AUC2; paclitaxel 50 mg/m2 day 1, 8, 15, 22, 29). Radiotherapy dose was 45 Gy/25 fractions/5 weeks. Both arms received induction OxCap chemotherapy (2 × 3 week cycles of oxaliplatin 130 mg/m2 day 1, capecitabine 625 mg/m2 bd days 1-21). Surgery was performed 6-8 weeks after nCRT. Primary end-point was pathological complete response (pCR). Secondary end-points included toxicity, surgical morbidity/mortality, resection rate and overall survival.
Statistics: Based on pCR ≤ 15% not warranting future investigation, but pCR ≥ 35% would, 76 patients (38/arm) gave 90% power (one-sided alpha 10%), implying that arm(s) having ≥10 pCR out of first 38 patients could be considered for phase III trials. ClinicalTrials.gov: NCT01843829. Funder: Cancer Research UK (C44694/A14614).
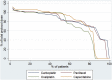
Results: Eighty five patients were randomised between October 2013 and February 2015 from 17 UK centres. Three of 85 (3.5%) died during induction chemotherapy. Seventy-seven patients (OxCapRT = 36; CarPacRT = 41) underwent surgery. The 30-d post-operative mortality was 2/77 (2.6%). Grade III/IV toxicity was comparable between arms, although neutropenia was higher in the CarPacRT arm (21.4% versus 2.6%, p = 0.01). Twelve of 41 (29.3%) (10 of first 38 patients) and 4/36 (11.1%) achieved pCR in the CarPacRT and OxcapRT arms, respectively. Corresponding R0 resection rates were 33/41 (80.5%) and 26/36 (72.2%), respectively.
Conclusion: Both regimens were well tolerated. Only CarPacRT passed the predefined pCR criteria for further investigation.
Keywords: Chemotherapy; Neo-adjuvant; Oesophageal; Radiotherapy; Randomised phase II; Surgery.
Copyright © 2017 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures
Comment in
-
Neoadjuvant chemoradiotherapy for oesophageal cancer: Still looking for a challenger to the CROSS regimen.Eur J Cancer. 2017 Sep;83:331-332. doi: 10.1016/j.ejca.2017.06.039. Epub 2017 Jul 20. Eur J Cancer. 2017. PMID: 28736189 No abstract available.
-
Response to: Neoadjuvant chemoradiotherapy for oesophageal cancer: Still looking for a challenger to the CROSS regimen.Eur J Cancer. 2017 Sep;83:333-334. doi: 10.1016/j.ejca.2017.06.040. Epub 2017 Jul 24. Eur J Cancer. 2017. PMID: 28751071 No abstract available.
References
-
- Urschel J.D., Vasan H. A meta-analysis of randomized controlled trials that compared neoadjuvant chemoradiation and surgery to surgery alone for resectable esophageal cancer. Am J Surg. 2003;185(6):538–543. - PubMed
-
- van Hagen P., Hulshof M.C., van Lanschot J.J., Steyerberg E.W., van Berge Henegouwen M.I., Wijnhoven B.P. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366(22):2074–2084. - PubMed
-
- Cunningham D., Allum W.H., Stenning S.P., Thompson J.N., Van de Velde C.J., Nicolson M. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11–20. - PubMed
-
- Cunningham D., Langley R., Nankivell M., Blazeby J., Griffin M., Crellin A. LBA-03 neoadjuvant chemotherapy for resectable oesophageal and junctional adenocarcinoma: results from the UK Medical Research Council randomised OEO5 trial (ISRCTN 01852072) Ann Oncol. 2015;26(Suppl. 4):iv117–iv118.
-
- Cunningham D., Smyth E., Stenning S., Stevenson L., Robb C., Allum W. 2201 Peri-operative chemotherapy±bevacizumab for resectable gastrooesophageal adenocarcinoma: results from the UK Medical Research Council randomised ST03 trial (ISRCTN 46020948) Eur J Cancer. 2015;51:S400.
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous