Cardiolipin and mitochondrial cristae organization
- PMID: 28336315
- PMCID: PMC5426559
- DOI: 10.1016/j.bbamem.2017.03.013
Cardiolipin and mitochondrial cristae organization
Abstract
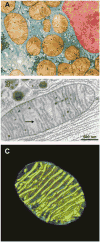
A fundamental question in cell biology, under investigation for over six decades, is the structural organization of mitochondrial cristae. Long known to harbor electron transport chain proteins, crista membrane integrity is key to establishment of the proton gradient that drives oxidative phosphorylation. Visualization of cristae morphology by electron microscopy/tomography has provided evidence that cristae are tube-like extensions of the mitochondrial inner membrane (IM) that project into the matrix space. Reconciling ultrastructural data with the lipid composition of the IM provides support for a continuously curved cylindrical bilayer capped by a dome-shaped tip. Strain imposed by the degree of curvature is relieved by an asymmetric distribution of phospholipids in monolayer leaflets that comprise cristae membranes. The signature mitochondrial lipid, cardiolipin (~18% of IM phospholipid mass), and phosphatidylethanolamine (34%) segregate to the negatively curved monolayer leaflet facing the crista lumen while the opposing, positively curved, matrix-facing monolayer leaflet contains predominantly phosphatidylcholine. Associated with cristae are numerous proteins that function in distinctive ways to establish and/or maintain their lipid repertoire and structural integrity. By combining unique lipid components with a set of protein modulators, crista membranes adopt and maintain their characteristic morphological and functional properties. Once established, cristae ultrastructure has a direct impact on oxidative phosphorylation, apoptosis, fusion/fission as well as diseases of compromised energy metabolism.
Keywords: Cardiolipin; Cristae; Electron transport chain; Membrane curvature; Mitochondria; Non-bilayer lipid; Phospholipid.
Copyright © 2017 Elsevier B.V. All rights reserved.
Figures
Similar articles
-
An evidence based hypothesis on the existence of two pathways of mitochondrial crista formation.Elife. 2016 Nov 16;5:e18853. doi: 10.7554/eLife.18853. Elife. 2016. PMID: 27849155 Free PMC article.
-
MICOS coordinates with respiratory complexes and lipids to establish mitochondrial inner membrane architecture.Elife. 2015 Apr 28;4:e07739. doi: 10.7554/eLife.07739. Elife. 2015. PMID: 25918844 Free PMC article.
-
Who and how in the regulation of mitochondrial cristae shape and function.Biochem Biophys Res Commun. 2018 May 27;500(1):94-101. doi: 10.1016/j.bbrc.2017.04.088. Epub 2017 Apr 21. Biochem Biophys Res Commun. 2018. PMID: 28438601 Review.
-
APOOL is a cardiolipin-binding constituent of the Mitofilin/MINOS protein complex determining cristae morphology in mammalian mitochondria.PLoS One. 2013 May 21;8(5):e63683. doi: 10.1371/journal.pone.0063683. Print 2013. PLoS One. 2013. PMID: 23704930 Free PMC article.
-
The role of nonbilayer phospholipids in mitochondrial structure and function.FEBS Lett. 2018 Apr;592(8):1273-1290. doi: 10.1002/1873-3468.12887. Epub 2017 Nov 9. FEBS Lett. 2018. PMID: 29067684 Free PMC article. Review.
Cited by
-
Effects of heat shock on photosynthesis-related characteristics and lipid profile of Cycas multipinnata and C. panzhihuaensis.BMC Plant Biol. 2022 Sep 15;22(1):442. doi: 10.1186/s12870-022-03825-0. BMC Plant Biol. 2022. PMID: 36109687 Free PMC article.
-
Plasmalogen loss caused by remodeling deficiency in mitochondria.Life Sci Alliance. 2019 Aug 21;2(4):e201900348. doi: 10.26508/lsa.201900348. Print 2019 Aug. Life Sci Alliance. 2019. PMID: 31434794 Free PMC article.
-
Phospholipid Membrane Transport and Associated Diseases.Biomedicines. 2022 May 23;10(5):1201. doi: 10.3390/biomedicines10051201. Biomedicines. 2022. PMID: 35625937 Free PMC article. Review.
-
Calcium-induced transformation of cardiolipin nanodisks.Biochim Biophys Acta Biomembr. 2019 May 1;1861(5):1030-1036. doi: 10.1016/j.bbamem.2019.03.005. Epub 2019 Mar 13. Biochim Biophys Acta Biomembr. 2019. PMID: 30876942 Free PMC article.
-
c-MYC Triggers Lipid Remodelling During Early Somatic Cell Reprogramming to Pluripotency.Stem Cell Rev Rep. 2021 Dec;17(6):2245-2261. doi: 10.1007/s12015-021-10239-2. Epub 2021 Sep 2. Stem Cell Rev Rep. 2021. PMID: 34476741 Free PMC article.
References
-
- Labbe K, Murley A, Nunnari J. Determinants and functions of mitochondrial behavior. Ann Rev Cell Develop Biol. 2014;30:357–391. - PubMed
-
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell. New York: Garland Publishing Inc; 1994.
-
- Voet D, Voet JG, Pratt CW. Fundamentals of Biochemistry. 2nd. John Wiley and Sons, Inc; 2006. pp. 547–556.
-
- Logan DC. The mitochondrial compartment. J Exp Bot. 2006;57:1225–1243. - PubMed
-
- Lea PJ, Hollenberg MJ. Mitochondrial structure revealed by high-resolution scanning electron microscopy. Am J Anat. 1989;184:245–257. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

