M1 muscarinic activation induces long-lasting increase in intrinsic excitability of striatal projection neurons
- PMID: 28336323
- PMCID: PMC5501169
- DOI: 10.1016/j.neuropharm.2017.03.017
M1 muscarinic activation induces long-lasting increase in intrinsic excitability of striatal projection neurons
Abstract
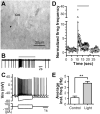
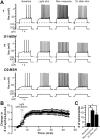
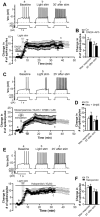
The dorsolateral striatum is critically involved in movement control and motor learning. Striatal function is regulated by a variety of neuromodulators including acetylcholine. Previous studies have shown that cholinergic activation excites striatal principal projection neurons, medium spiny neurons (MSNs), and this action is mediated by muscarinic acetylcholine subtype 1 receptors (M1) through modulating multiple potassium channels. In the present study, we used electrophysiology techniques in conjunction with optogenetic and pharmacological tools to determine the long-term effects of striatal cholinergic activation on MSN intrinsic excitability. A transient increase in acetylcholine release in the striatum by optogenetic stimulation resulted in a long-lasting increase in excitability of MSNs, which was associated with hyperpolarizing shift of action potential threshold and decrease in afterhyperpolarization (AHP) amplitude, leading to an increase in probability of EPSP-action potential coupling. The M1 selective antagonist VU0255035 prevented, while the M1 selective positive allosteric modulator (PAM) VU0453595 potentiated the cholinergic activation-induced persistent increase in MSN intrinsic excitability, suggesting that M1 receptors are critically involved in the induction of this long-lasting response. This M1 receptor-dependent long-lasting change in MSN intrinsic excitability could have significant impact on striatal processing and might provide a novel mechanism underlying cholinergic regulation of the striatum-dependent motor learning and cognitive function. Consistent with this, behavioral studies indicate that potentiation of M1 receptor signaling by VU0453595 enhanced performance of mice in cue-dependent water-based T-maze, a dorsolateral striatum-dependent learning task.
Keywords: AP-EPSP coupling; Dorsolateral striatum-dependent learning; Intrinsic plasticity; M(1) muscarinic receptor; Striatal MSNs.
Copyright © 2017. Published by Elsevier Ltd.
Conflict of interest statement
J.M.R., C.W.L. and P.J.C. are inventors on multiple composition of matter patents protecting allosteric modulators of M1.
Figures





Similar articles
-
Involvement of Striatal Cholinergic Interneurons and M1 and M4 Muscarinic Receptors in Motor Symptoms of Parkinson's Disease.J Neurosci. 2016 Aug 31;36(35):9161-72. doi: 10.1523/JNEUROSCI.0873-16.2016. J Neurosci. 2016. PMID: 27581457 Free PMC article.
-
Neostriatal GABAergic Interneurons Mediate Cholinergic Inhibition of Spiny Projection Neurons.J Neurosci. 2016 Sep 7;36(36):9505-11. doi: 10.1523/JNEUROSCI.0466-16.2016. J Neurosci. 2016. PMID: 27605623 Free PMC article.
-
Cell-Specific Cholinergic Modulation of Excitability of Layer 5B Principal Neurons in Mouse Auditory Cortex.J Neurosci. 2016 Aug 10;36(32):8487-99. doi: 10.1523/JNEUROSCI.0780-16.2016. J Neurosci. 2016. PMID: 27511019 Free PMC article.
-
Cholinergic control of striatal neurons to modulate L-dopa-induced dyskinesias.Eur J Neurosci. 2019 Mar;49(6):859-868. doi: 10.1111/ejn.14048. Epub 2018 Jul 21. Eur J Neurosci. 2019. PMID: 29923650 Review.
-
Cholinergic modulation of synaptic integration and dendritic excitability in the striatum.Curr Opin Neurobiol. 2011 Jun;21(3):425-32. doi: 10.1016/j.conb.2011.04.004. Curr Opin Neurobiol. 2011. PMID: 21550798 Free PMC article. Review.
Cited by
-
Pauses in cholinergic interneuron firing exert an inhibitory control on striatal output in vivo.Elife. 2018 Mar 26;7:e32510. doi: 10.7554/eLife.32510. Elife. 2018. PMID: 29578407 Free PMC article.
-
Striatal Dopamine D2-Muscarinic Acetylcholine M1 Receptor-Receptor Interaction in a Model of Movement Disorders.Front Pharmacol. 2020 Mar 13;11:194. doi: 10.3389/fphar.2020.00194. eCollection 2020. Front Pharmacol. 2020. PMID: 32231561 Free PMC article.
-
Cholinergic modulation of striatal microcircuits.Eur J Neurosci. 2019 Mar;49(5):604-622. doi: 10.1111/ejn.13949. Epub 2018 Nov 29. Eur J Neurosci. 2019. PMID: 29797362 Free PMC article. Review.
-
Biased M1 receptor-positive allosteric modulators reveal role of phospholipase D in M1-dependent rodent cortical plasticity.Sci Signal. 2019 Dec 3;12(610):eaax2057. doi: 10.1126/scisignal.aax2057. Sci Signal. 2019. PMID: 31796631 Free PMC article.
-
Modulation of arousal and sleep/wake architecture by M1 PAM VU0453595 across young and aged rodents and nonhuman primates.Neuropsychopharmacology. 2020 Dec;45(13):2219-2228. doi: 10.1038/s41386-020-00812-7. Epub 2020 Aug 31. Neuropsychopharmacology. 2020. PMID: 32868847 Free PMC article.
References
-
- Akins PT, Surmeier DJ, Kitai ST. M1 muscarinic acetylcholine receptor in cultured rat neostriatum regulates phosphoinositide hydrolysis. J Neurochem. 1990a;54:266–273. - PubMed
-
- Akins PT, Surmeier DJ, Kitai ST. Muscarinic modulation of a transient K+ conductance in rat neostriatal neurons. Nature. 1990b;344:240–242. - PubMed
-
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. - PubMed
-
- Aosaki T, Kimura M, Graybiel AM. Temporal and spatial characteristics of tonically active neurons of the primate’s striatum. J Neurophysiol. 1995;73:1234–1252. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

