A phase 1 clinical trial of single-agent selinexor in acute myeloid leukemia
- PMID: 28336527
- PMCID: PMC5524530
- DOI: 10.1182/blood-2016-11-750158
A phase 1 clinical trial of single-agent selinexor in acute myeloid leukemia
Erratum in
-
Garzon R, Savona M, Baz R, et al. A phase 1 clinical trial of single-agent selinexor in acute myeloid leukemia. Blood. 2017;129(24):3165-3174.Blood. 2020 Jul 9;136(2):259. doi: 10.1182/blood.2020007231. Blood. 2020. PMID: 32645169 Free PMC article. No abstract available.
Abstract
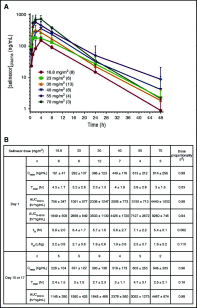
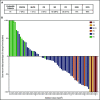
Selinexor is a novel, first-in-class, selective inhibitor of nuclear export compound, which blocks exportin 1 (XPO1) function, leads to nuclear accumulation of tumor suppressor proteins, and induces cancer cell death. A phase 1 dose-escalation study was initiated to examine the safety and efficacy of selinexor in patients with advanced hematological malignancies. Ninety-five patients with relapsed or refractory acute myeloid leukemia (AML) were enrolled between January 2013 and June 2014 to receive 4, 8, or 10 doses of selinexor in a 21- or 28-day cycle. The most frequently reported adverse events (AEs) in patients with AML were grade 1 or 2 constitutional and gastrointestinal toxicities, which were generally manageable with supportive care. The only nonhematological grade 3/4 AE, occurring in >5% of the patient population, was fatigue (14%). There were no reported dose-limiting toxicities or evidence of cumulative toxicity. The recommended phase 2 dose was established at 60 mg (∼35 mg/m2) given twice weekly in a 4-week cycle based on the totality of safety and efficacy data. Overall, 14% of the 81 evaluable patients achieved an objective response (OR) and 31% percent showed ≥50% decrease in bone marrow blasts from baseline. Patients achieving an OR had a significant improvement in median progression-free survival (PFS) (5.1 vs 1.3 months; P = .008; hazard ratio [HR], 3.1) and overall survival (9.7 vs 2.7 months; P = .01; HR, 3.1) compared with nonresponders. These findings suggest that selinexor is safe as a monotherapy in patients with relapsed or refractory AML and have informed subsequent phase 2 clinical development. This trial was registered at www.clinicaltrials.gov as #NCT01607892.
© 2017 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: R.G. received a travel stipend from Karyopharm Therapeutics. M.S. owns stock in Karyopharm Therapeutics, has a consulting/advisory role at Amgen, CTI, Karyopharm Therapeutics, ARIAD, Gilead, and Celgene, and receives research funding from Astex, Sunesis, Takeda, and TG Therapeutics. R.B. has a consulting/advisory role at Celgene and receives research funding from Celgene, Karyopharm Therapeutics, Merck, Bristol-Myers Squibb, and Millennium/Takeda. N.G. receives honoraria from Heron, Sanofi, and Taiho and is a consultant/advisor at Heron. L.S. has a consulting/advisory role at Novartis, Bristol-Myers Squibb, Pfizer, Celgene, and JAZZ. P.M.M.-S. receives honorarium and a travel stipend from Karyopharm Therapeutics. T.J.U., J.-R.S.-M., R.C., T.R., T.K., B.K., S.S., and M.K. are employees and stockholders at Karyopharm Therapeutics. R.S. receives honoraria from Amgen, Arog, Agios, Celfor, Celgene, Sunesis, Bristol-Myers Squibb, Pfizer, Novartis, Karyopharm Therapeutics, Fujifilm, AbbVie, Roche, Genentech, and Merck, and receives research funding from Novartis. The remaining authors declare no competing financial interests.
Figures


Comment in
-
Restoring leukemia cell function from the inside out.Blood. 2017 Jun 15;129(24):3137-3138. doi: 10.1182/blood-2017-04-776344. Blood. 2017. PMID: 28620100 No abstract available.
References
-
- Grimwade D, Walker H, Oliver F, et al. ; The Medical Research Council Adult and Children’s Leukaemia Working Parties . The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. Blood. 1998;92(7):2322-2333. - PubMed
-
- Whitman SP, Archer KJ, Feng L, et al. Absence of the wild-type allele predicts poor prognosis in adult de novo acute myeloid leukemia with normal cytogenetics and the internal tandem duplication of FLT3: a cancer and leukemia group B study. Cancer Res. 2001;61(19):7233-7239. - PubMed
-
- Falini B, Mecucci C, Tiacci E, et al. ; GIMEMA Acute Leukemia Working Party . Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N Engl J Med. 2005;352(3):254-266. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

