Lysosomal cholesterol activates mTORC1 via an SLC38A9-Niemann-Pick C1 signaling complex
- PMID: 28336668
- PMCID: PMC5823611
- DOI: 10.1126/science.aag1417
Lysosomal cholesterol activates mTORC1 via an SLC38A9-Niemann-Pick C1 signaling complex
Abstract
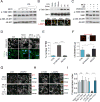
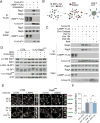
The mechanistic target of rapamycin complex 1 (mTORC1) protein kinase is a master growth regulator that becomes activated at the lysosome in response to nutrient cues. Here, we identify cholesterol, an essential building block for cellular growth, as a nutrient input that drives mTORC1 recruitment and activation at the lysosomal surface. The lysosomal transmembrane protein, SLC38A9, is required for mTORC1 activation by cholesterol through conserved cholesterol-responsive motifs. Moreover, SLC38A9 enables mTORC1 activation by cholesterol independently from its arginine-sensing function. Conversely, the Niemann-Pick C1 (NPC1) protein, which regulates cholesterol export from the lysosome, binds to SLC38A9 and inhibits mTORC1 signaling through its sterol transport function. Thus, lysosomal cholesterol drives mTORC1 activation and growth signaling through the SLC38A9-NPC1 complex.
Copyright © 2017, American Association for the Advancement of Science.
Figures


References
-
- Nohturfft A, Zhang SC. Coordination of lipid metabolism in membrane biogenesis. Annual review of cell and developmental biology. 2009;25:539–566. - PubMed
-
- Espenshade PJ, Hughes AL. Regulation of sterol synthesis in eukaryotes. Annual review of genetics. 2007;41:401–427. - PubMed
-
- Kalaany NY, Mangelsdorf DJ. LXRS and FXR: the yin and yang of cholesterol and fat metabolism. Annual review of physiology. 2006;68:159–191. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

