Disparities between research attention and burden in liver diseases: implications on uneven advances in pharmacological therapies in Europe and the USA
- PMID: 28336739
- PMCID: PMC5372160
- DOI: 10.1136/bmjopen-2016-013620
Disparities between research attention and burden in liver diseases: implications on uneven advances in pharmacological therapies in Europe and the USA
Abstract
Objectives: Effective oral therapies for hepatitis B and C have recently been developed, while there are no approved pharmacological therapies for alcoholic and non-alcoholic fatty liver diseases (ALD and NAFLD). We hypothesise that fewer advances in fatty liver diseases could be related to disparities in research attention.
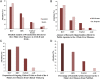
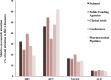
Methods: We developed the Attention-to-Burden Index (ABI) that compares the research activities during 2010-2014, and an estimate of disease burden of these 4 major liver diseases. The resulting ratio reflects either overattention (positive value) or inadequate attention (negative value) compared with disease burden. The mean research attention and disease burden were calculated from 5 and 6 different parameters, respectively. The efficacy rate of current pharmacological therapies was assessed from published clinical trials.
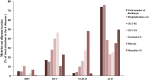
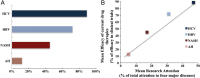
Findings: The mean research attention for hepatitis B and C was 31% and 47%, respectively, while NAFLD and ALD received 17% and 5%. The overall burden was 5% and 28% for hepatitis B and C, and 17% and 50% for NAFLD and ALD. The calculated ABI for hepatitis B and C revealed a +6.7-fold and +1.7-fold overattention, respectively. NAFLD received an appropriate attention compared with its burden, while ALD received marked inadequate attention of -9.7-fold. The efficacy rate of current pharmacological agents was 72% for hepatitis B, 89% for hepatitis C, 25% for non-alcoholic steatohepatitis and 13% for alcoholic hepatitis. Importantly, we found a positive correlation between the mean attention and the efficacy rate of current therapies in these 4 major liver diseases.
Interpretation: There are important disparities between research attention and disease burden among the major liver diseases. While viral hepatitis has received considerable attention, there is a marked inadequate attention to ALD. There is a critical need to increase awareness of ALD in the liver research community.
Keywords: Hepatitis B virus; Hepatitis C virus; Non-alcoholic fatty liver disease; PUBLIC HEALTH; alcoholic liver disease.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/.
Conflict of interest statement
Figures

References
-
- Poznyak V, Rekve D. Global status report on alcohol and health 2014. World Health Organization, 2014.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
