Role of transient receptor potential vanilloid 1 in the modulation of airway smooth muscle tone and calcium handling
- PMID: 28336810
- PMCID: PMC5495950
- DOI: 10.1152/ajplung.00064.2017
Role of transient receptor potential vanilloid 1 in the modulation of airway smooth muscle tone and calcium handling
Abstract
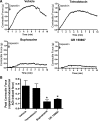
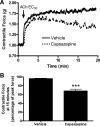
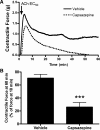
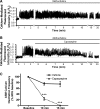
Asthma is a common disorder characterized, in part, by airway smooth muscle (ASM) hyperresponsiveness. Transient receptor potential vanilloid 1 (TRPV1) is a nonselective cation channel expressed on airway nerve fibers that modulates afferent signals, resulting in cough, and potentially bronchoconstriction. In the present study, the TRPV1 transcript was detected by RT-PCR in primary cultured human ASM cells, and the TRPV1 protein was detected in ASM of human trachea by immunohistochemistry. Proximity ligation assays suggest that TRPV1 is expressed in the sarcoplasmic reticulum membrane of human ASM cells in close association with sarco/endoplasmic reticulum Ca2+-ATPase-2. In guinea pig tracheal ring organ bath experiments, the TRPV1 agonist capsaicin led to ASM contraction, but this contraction was significantly attenuated by the sodium channel inhibitor bupivacaine (n = 4, P < 0.05) and the neurokinin-2 receptor antagonist GR-159897 (n = 4, P < 0.05), suggesting that this contraction is neutrally mediated. However, pretreatment of guinea pig and human ASM in organ bath experiments with the TRPV1 antagonist capsazepine inhibited the maintenance phase of an acetylcholine-induced contraction (n = 4, P < 0.01 for both species). Similarly, capsazepine inhibited methacholine-induced contraction of peripheral airways in mouse precision-cut lung slice (PCLS) experiments (n = 4-5, P < 0.05). Although capsazepine did not inhibit store-operated calcium entry in mouse ASM cells in PCLS (n = 4-7, P = nonsignificant), it did inhibit calcium oscillations (n = 3, P < 0.001). These studies suggest that TRPV1 is expressed on ASM, including the SR, but that ASM TRPV1 activation does not play a significant role in initiation of ASM contraction. However, capsazepine does inhibit maintenance of contraction, likely by inhibiting calcium oscillations.
Keywords: asthma; calcium oscillations; capsazepine; organ bath; precision-cut lung slice.
Copyright © 2017 the American Physiological Society.
Figures






Similar articles
-
Capsazepine, a vanilloid antagonist, abolishes tonic responses induced by 20-HETE on guinea pig airway smooth muscle.Am J Physiol Lung Cell Mol Physiol. 2005 Mar;288(3):L460-70. doi: 10.1152/ajplung.00252.2004. Epub 2004 Nov 19. Am J Physiol Lung Cell Mol Physiol. 2005. PMID: 15557084
-
Agonism of the TMEM16A calcium-activated chloride channel modulates airway smooth muscle tone.Am J Physiol Lung Cell Mol Physiol. 2020 Feb 1;318(2):L287-L295. doi: 10.1152/ajplung.00552.2018. Epub 2019 Nov 20. Am J Physiol Lung Cell Mol Physiol. 2020. PMID: 31747299 Free PMC article.
-
Antagonists of the TMEM16A calcium-activated chloride channel modulate airway smooth muscle tone and intracellular calcium.Anesthesiology. 2015 Sep;123(3):569-81. doi: 10.1097/ALN.0000000000000769. Anesthesiology. 2015. PMID: 26181339 Free PMC article.
-
Ionic mechanisms and Ca(2+) regulation in airway smooth muscle contraction: do the data contradict dogma?Am J Physiol Lung Cell Mol Physiol. 2002 Jun;282(6):L1161-78. doi: 10.1152/ajplung.00452.2001. Am J Physiol Lung Cell Mol Physiol. 2002. PMID: 12003770 Review.
-
Calcium Entry through TRPV1: A Potential Target for the Regulation of Proliferation and Apoptosis in Cancerous and Healthy Cells.Int J Mol Sci. 2020 Jun 11;21(11):4177. doi: 10.3390/ijms21114177. Int J Mol Sci. 2020. PMID: 32545311 Free PMC article. Review.
Cited by
-
TRPPing up bronchiectasis.Am J Physiol Lung Cell Mol Physiol. 2019 Oct 1;317(4):L464-L465. doi: 10.1152/ajplung.00363.2019. Epub 2019 Sep 11. Am J Physiol Lung Cell Mol Physiol. 2019. PMID: 31508977 Free PMC article. No abstract available.
-
Estrogenic Modulation of Ionic Channels, Pumps and Exchangers in Airway Smooth Muscle.Int J Mol Sci. 2023 Apr 26;24(9):7879. doi: 10.3390/ijms24097879. Int J Mol Sci. 2023. PMID: 37175587 Free PMC article. Review.
-
A novel GABAA receptor ligand MIDD0301 with limited blood-brain barrier penetration relaxes airway smooth muscle ex vivo and in vivo.Am J Physiol Lung Cell Mol Physiol. 2019 Feb 1;316(2):L385-L390. doi: 10.1152/ajplung.00356.2018. Epub 2018 Nov 29. Am J Physiol Lung Cell Mol Physiol. 2019. PMID: 30489155 Free PMC article.
-
Chamaecyparis lawsoniana and Its Active Compound Quercetin as Ca2+ Inhibitors in the Contraction of Airway Smooth Muscle.Molecules. 2024 May 12;29(10):2284. doi: 10.3390/molecules29102284. Molecules. 2024. PMID: 38792145 Free PMC article.
-
Transient Receptor Potential Channels and Chronic Airway Inflammatory Diseases: A Comprehensive Review.Lung. 2018 Oct;196(5):505-516. doi: 10.1007/s00408-018-0145-3. Epub 2018 Aug 9. Lung. 2018. PMID: 30094794 Review.
References
-
- Cantero-Recasens G, Gonzalez JR, Fandos C, Duran-Tauleria E, Smit LA, Kauffmann F, Antó JM, Valverde MA. Loss of function of transient receptor potential vanilloid 1 (TRPV1) genetic variant is associated with lower risk of active childhood asthma. J Biol Chem 285: 27532–27535, 2010. doi:10.1074/jbc.C110.159491. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

