Identification and Evaluation of New Immunoregulatory Genes in Mesenchymal Stromal Cells of Different Origins: Comparison of Normal and Inflammatory Conditions
- PMID: 28336906
- PMCID: PMC5378277
- DOI: 10.12659/msmbr.903518
Identification and Evaluation of New Immunoregulatory Genes in Mesenchymal Stromal Cells of Different Origins: Comparison of Normal and Inflammatory Conditions
Abstract
BACKGROUND Mesenchymal stromal cells (MSCs) possess potent immunomodulatory properties that increase their value as a cell-based therapeutic tool for managing various immune-based disorders. Over the past years, accumulated results from trials using MSCs-based therapy have shown substantial contradictions. Although the reasons underlying these discrepancies are still not completely understood, it is well known that the immunomodulatory activities mediated by distinct MSCs differ in a manner dependent on their tissue origin and adequate response to inflammation priming. Thus, characterization of new molecular pathway(s) through which distinct MSC populations can exert their immunomodulatory effects, particularly during inflammation, will undoubtedly enhance their therapeutic potential. MATERIAL AND METHODS After confirming their compliance with ISCT criteria, quantitative real time-PCR (qRT-PCR) was used to screen new immunoregulatory genes in MSCs, derived from adipose tissue, foreskin, Wharton's jelly or the bone-marrow, after being cultivated under normal and inflammatory conditions. RESULTS FGL2, GAL, SEMA4D, SEMA7A, and IDO1 genes appeared to be differentially transcribed in the different MSC populations. Moreover, these genes were not similarly modulated following MSCs-exposure to inflammatory signals. CONCLUSIONS Our observations suggest that these identified immunoregulatory genes may be considered as potential candidates to be targeted in order to enhance the immunomodulatory properties of MSCs towards more efficient clinical use.
Figures
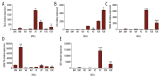
References
-
- Friedenstein AJ, Deriglasova UF, Kulagina NN, et al. Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp Hematol. 1974;2:83–92. - PubMed
-
- Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–47. - PubMed
-
- Bartholomew A, Sturgeon C, Siatskas M, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42–48. - PubMed
-
- Zappia E, Casazza S, Pedemonte E, et al. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005;106:1755–61. - PubMed
-
- Le Blanc K, Rasmusson I, Sundberg B, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet (London, England) 2004;363:1439–41. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

