Neuronal Mitophagy in Neurodegenerative Diseases
- PMID: 28337125
- PMCID: PMC5340781
- DOI: 10.3389/fnmol.2017.00064
Neuronal Mitophagy in Neurodegenerative Diseases
Abstract
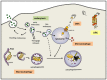
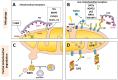
Neuronal homeostasis depends on the proper functioning of different quality control systems. All intracellular components are subjected to continuous turnover through the coordinated synthesis, degradation and recycling of their constituent elements. Autophagy is the catabolic mechanism by which intracellular cytosolic components, including proteins, organelles, aggregates and any other intracellular materials, are delivered to lysosomes for degradation. Among the different types of selective autophagy described to date, the process of mitophagy involves the selective autophagic degradation of mitochondria. In this way, mitophagy is responsible for basal mitochondrial turnover, but can also be induced under certain physiological or pathogenic conditions to eliminate unwanted or damaged mitochondria. Dysfunctional cellular proteolytic systems have been linked extensively to neurodegenerative diseases (ND) like Alzheimer's disease (AD), Parkinson's disease (PD), or Huntington's disease (HD), with autophagic failure being one of the main factors contributing to neuronal cell death in these diseases. Neurons are particularly vulnerable to autophagic impairment as well as to mitochondrial dysfunction, due mostly to their particular high energy dependence and to their post-mitotic nature. The accurate and proper degradation of dysfunctional mitochondria by mitophagy is essential for maintaining control over mitochondrial quality and quantity in neurons. In this report, I will review the role of mitophagy in neuronal homeostasis and the consequences of its dysfunction in ND.
Keywords: Alzheimer disease; Parkinson’s disease; autophagy; mitophagy; neurodegeneration.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

