Bone Marrow-Derived Cell Accumulation in the Spinal Cord Is Independent of Peripheral Mobilization in a Mouse Model of Amyotrophic Lateral Sclerosis
- PMID: 28337172
- PMCID: PMC5340765
- DOI: 10.3389/fneur.2017.00075
Bone Marrow-Derived Cell Accumulation in the Spinal Cord Is Independent of Peripheral Mobilization in a Mouse Model of Amyotrophic Lateral Sclerosis
Abstract
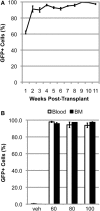
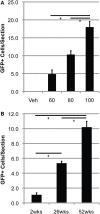
Bone marrow-derived cells (BMDCs) are capable of migrating across the blood-brain barrier (BBB) and accumulating in the central nervous system (CNS) when transplanted into recipients conditioned with whole-body irradiation or chemotherapy. We used the chemotherapeutic agents busulfan and treosulfan to condition recipient mice for transplantation with bone marrow (BM) cells isolated from donor mice ubiquitously expressing green fluorescent protein. We attempted to increase the accumulation of BMDCs in the CNS by mobilization of BMDCs using either, or both, granulocyte colony-stimulating factor (GCSF) or plerixafor (AMD3100). We also used several concentrations of busulfan. We hypothesized that higher concentrations of busulfan and BMDC mobilization would increase numbers of GFP+ cells in the CNS. The doses of busulfan employed (60-125 mg/kg) all resulted in high levels of sustained chimerism (>85% 1 year post-transplant) in both the blood and BM of wild-type (WT) mice and an amyotrophic lateral sclerosis (ALS) mouse model. Moreover, cells accumulated within the CNS in a dose-, time-, and disease-dependent manner. Conditioning with the hydrophilic busulfan analog treosulfan, which is unable to cross the BBB efficiently, also resulted in a high degree of BM chimerism. However, few GFP+ BMDCs were found within the CNS of WT or ALS mice of treosulfan-conditioned mice. Mobilization of BMDCs into the circulation using GCSF and/or AMD3100 did not lead to increased accumulation of GFP+ BMDCs within the CNS of WT or ALS mice. Weekly analysis of BMDC accumulation revealed that BMDCs accumulated more rapidly and to a greater extent in the CNS of ALS mice conditioned with a high dose (125 mg/kg) of busulfan compared to a lower dose (80 mg/kg). The number of GFP+ BMDCs in the CNS labeling with the proliferation marker Ki67 increased in parallel with BMDC accumulation within the CNS. Our results indicate that establishment of high levels of blood and BM chimerism alone is not sufficient to induce BMDC accumulation within the CNS and that CNS conditioning is a crucial requirement for BMDC accumulation to occur. Moreover, it appears that proliferation of BMDCs that infiltrate the CNS is partly responsible for cell accumulation in busulfan-conditioned ALS mice.
Keywords: AMD3100; amyotrophic lateral sclerosis; bone marrow-derived cells; busulfan; central nervous system; granulocyte colony-stimulating factor; monocyte; treosulfan.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

