NK Cells: Uncertain Allies against Malaria
- PMID: 28337195
- PMCID: PMC5343013
- DOI: 10.3389/fimmu.2017.00212
NK Cells: Uncertain Allies against Malaria
Abstract
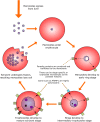
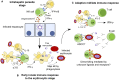
Until recently, studies of natural killer (NK) cells in infection have focused almost entirely on their role in viral infections. However, there is an increasing awareness of the potential for NK cells to contribute to the control of a wider range of pathogens, including intracellular parasites such as Plasmodium spp. Given the high prevalence of parasitic diseases in the developing world and the devastating effects these pathogens have on large numbers of vulnerable people, investigating interactions between NK cells and parasitized host cells presents the opportunity to reveal novel immunological mechanisms with the potential to aid efforts to eradicate these diseases. The capacity of NK cells to produce inflammatory cytokines early after malaria infection, as well as a possible role in direct cytotoxic killing of malaria-infected cells, suggests a beneficial impact of NK cells in this disease. However, NK cells may also contribute to overproduction of pro-inflammatory cytokines and the consequent immunopathology. As comparatively little is known about the role of NK cells later in the course of infection, and growing evidence suggests that heterogeneity in NK cell responses to malaria may be influenced by KIR/HLA interactions, a better understanding of the mechanisms by which NK cells might directly interact with parasitized cells may reveal a new role for these cells in the course of malaria infection.
Keywords: KIR; NK cells; cytotoxic killing; inflammatory cytokines; malaria.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

