Role of Metallic Nanoparticles in Vaccinology: Implications for Infectious Disease Vaccine Development
- PMID: 28337198
- PMCID: PMC5340775
- DOI: 10.3389/fimmu.2017.00239
Role of Metallic Nanoparticles in Vaccinology: Implications for Infectious Disease Vaccine Development
Abstract
Subunit vaccines are safer but less immunogenic than live-attenuated vaccines or whole cell inactivated vaccines. Adjuvants are used to enhance and modulate antigen (Ag) immunogenicity, aiming to induce a protective and long-lasting immune response. Several molecules and formulations have been studied for their adjuvanticity, but only seven have been approved to formulate human vaccines. Metallic nanoparticles (MeNPs), particularly those containing gold and iron oxides, are widely used in medicine for diagnosis and therapy and have been used as carriers for drugs and vaccines. However, little is known about the immune response elicited by MeNPs or about their importance in the development of new vaccines. There is evidence that these particles display adjuvant characteristics, promoting cell recruitment, antigen-presenting cell activation, cytokine production, and inducing a humoral immune response. This review focuses on the characteristics of MeNPs that could facilitate the induction of a cellular immune response, particularly T-helper 1 and T-helper 17, and their potential functions as adjuvants for subunit vaccines.
Keywords: Th1; Th17; adjuvant; immune response; particulate vaccine.
Figures
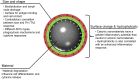
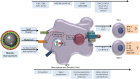
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

