Nutrient and Metabolic Sensing in T Cell Responses
- PMID: 28337199
- PMCID: PMC5343023
- DOI: 10.3389/fimmu.2017.00247
Nutrient and Metabolic Sensing in T Cell Responses
Abstract
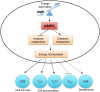
T cells play pivotal roles in shaping host immune responses in infectious diseases, autoimmunity, and cancer. The activation of T cells requires immune and growth factor-derived signals. However, alterations in nutrients and metabolic signals tune T cell responses by impinging upon T cell fates and immune functions. In this review, we summarize how key nutrients, including glucose, amino acids, and lipids, and their sensors and transporters shape T cell responses. We also briefly discuss regulation of T cell responses by oxygen and energy sensing mechanisms.
Keywords: T cell response; amino acid; energy; glucose; lipid; oxygen.
Figures
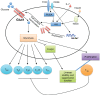
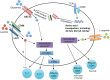
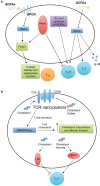
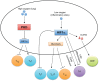
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

