Metabolic Hallmarks of Tumor and Immune Cells in the Tumor Microenvironment
- PMID: 28337200
- PMCID: PMC5340776
- DOI: 10.3389/fimmu.2017.00248
Metabolic Hallmarks of Tumor and Immune Cells in the Tumor Microenvironment
Abstract
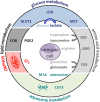
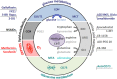
Cytotoxic T lymphocytes and NK cells play an important role in eliminating malignant tumor cells and the number and activity of tumor-infiltrating T cells represent a good marker for tumor prognosis. Based on these findings, immunotherapy, e.g., checkpoint blockade, has received considerable attention during the last couple of years. However, for the majority of patients, immune control of their tumors is gray theory as malignant cells use effective mechanisms to outsmart the immune system. Increasing evidence suggests that changes in tumor metabolism not only ensure an effective energy supply and generation of building blocks for tumor growth but also contribute to inhibition of the antitumor response. Immunosuppression in the tumor microenvironment is often based on the mutual metabolic requirements of immune cells and tumor cells. Cytotoxic T and NK cell activation leads to an increased demand for glucose and amino acids, a well-known feature shown by tumor cells. These close metabolic interdependencies result in metabolic competition, limiting the proliferation, and effector functions of tumor-specific immune cells. Moreover, not only nutrient restriction but also tumor-driven shifts in metabolite abundance and accumulation of metabolic waste products (e.g., lactate) lead to local immunosuppression, thereby facilitating tumor progression and metastasis. In this review, we describe the metabolic interplay between immune cells and tumor cells and discuss tumor cell metabolism as a target structure for cancer therapy. Metabolic (re)education of tumor cells is not only an approach to kill tumor cells directly but could overcome metabolic immunosuppression in the tumor microenvironment and thereby facilitate immunotherapy.
Keywords: cytokines; glycolysis and oxidative phosphorylation; immune cell functions; immune cell metabolism; immune escape; tumor metabolism.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

