Gut Mesenchymal Stromal Cells in Immunity
- PMID: 28337224
- PMCID: PMC5350335
- DOI: 10.1155/2017/8482326
Gut Mesenchymal Stromal Cells in Immunity
Abstract
Mesenchymal stromal cells (MSCs), first found in bone marrow (BM), are the structural architects of all organs, participating in most biological functions. MSCs possess tissue-specific signatures that allow their discrimination according to their origin and location. Among their multiple functions, MSCs closely interact with immune cells, orchestrating their activity to maintain overall homeostasis. The phenotype of tissue MSCs residing in the bowel overlaps with myofibroblasts, lining the bottom walls of intestinal crypts (pericryptal) or interspersed within intestinal submucosa (intercryptal). In Crohn's disease, intestinal MSCs are tightly stacked in a chronic inflammatory milieu, which causes their enforced expression of Class II major histocompatibility complex (MHC). The absence of Class II MHC is a hallmark for immune-modulator and tolerogenic properties of normal MSCs and, vice versa, the expression of HLA-DR is peculiar to antigen presenting cells, that is, immune-activator cells. Interferon gamma (IFNγ) is responsible for induction of Class II MHC expression on intestinal MSCs. The reversal of myofibroblasts/MSCs from an immune-modulator to an activator phenotype in Crohn's disease results in the formation of a fibrotic tube subverting the intestinal structure. Epithelial metaplastic areas in this context can progress to dysplasia and cancer.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
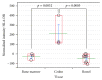
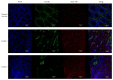
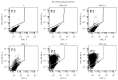
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

