Bifunctional Inhibitors as a New Tool To Reduce Cancer Cell Invasion by Impairing MMP-9 Homodimerization
- PMID: 28337319
- PMCID: PMC5346986
- DOI: 10.1021/acsmedchemlett.6b00446
Bifunctional Inhibitors as a New Tool To Reduce Cancer Cell Invasion by Impairing MMP-9 Homodimerization
Abstract
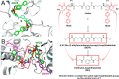
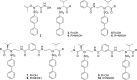
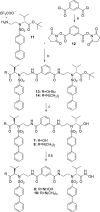
Protein homodimers play important roles in physiological and pathological processes, including cancer invasion and metastasis. Recently, MMP-9 natural homodimerization via the PEX domain has been correlated with high migration rates of aggressive cancer cells. Here we propose that bifunctional MMP-9 inhibitors designed to impair natural MMP-9 homodimerization promoted by PEX-PEX interactions might be an effective tool to fight cancer cell invasion. Elaborating a previously described dimeric hydroxamate inhibitor 1, new ligands were synthesized with different linker lengths and branch points. Evaluation of the modified bifunctional ligands by X-ray crystallography and biological assays showed that 7 and 8 could reduce invasion in three glioma cell lines expressing MMP-9 at different levels. To rationalize these results, we present a theoretical model of full-length MMP-9 in complex with 7. This pioneering study suggests that a new approach using MMP-9 selective bifunctional inhibitors might lead to an effective therapy to reduce cancer cell invasion.
Keywords: Bifunctional inhibitors; MMP-9 homodimerization; X-ray crystallography; glioblastoma multiforme.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

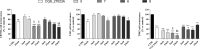

Similar articles
-
The intranuclear PEX domain of MMP involves proliferation, migration, and metastasis of aggressive adenocarcinoma cells.J Cell Biochem. 2018 Sep;119(9):7363-7376. doi: 10.1002/jcb.27040. Epub 2018 May 15. J Cell Biochem. 2018. PMID: 29761931
-
Creation of an apoptin-derived peptide that interacts with SH3 domains and inhibits glioma cell migration and invasion.Tumour Biol. 2016 Nov;37(11):15229-15240. doi: 10.1007/s13277-016-5404-4. Epub 2016 Sep 29. Tumour Biol. 2016. PMID: 27686608
-
Inhibition of membrane-type 1 matrix metalloproteinase at cell-matrix adhesions.Cancer Res. 2007 Dec 15;67(24):11621-9. doi: 10.1158/0008-5472.CAN-07-5251. Cancer Res. 2007. PMID: 18089791
-
Electrochemical impedance spectroscopy for quantization of matrix Metalloproteinase-14 based on peptides inhibiting its homodimerization and heterodimerization.Talanta. 2019 Dec 1;205:120142. doi: 10.1016/j.talanta.2019.120142. Epub 2019 Jul 18. Talanta. 2019. PMID: 31450394 Review.
-
The role of matrix metalloproteinases in glioma invasion.Front Biosci. 2003 Jan 1;8:e261-9. doi: 10.2741/1016. Front Biosci. 2003. PMID: 12456313 Review.
Cited by
-
Discovery of Dimeric Arylsulfonamides as Potent ADAM8 Inhibitors.ACS Med Chem Lett. 2021 Oct 8;12(11):1787-1793. doi: 10.1021/acsmedchemlett.1c00411. eCollection 2021 Nov 11. ACS Med Chem Lett. 2021. PMID: 35111280 Free PMC article.
-
Bacterial Zinc Metalloenzyme Inhibitors: Recent Advances and Future Perspectives.Molecules. 2023 May 27;28(11):4378. doi: 10.3390/molecules28114378. Molecules. 2023. PMID: 37298854 Free PMC article. Review.
-
Bivalent Inhibitor with Selectivity for Trimeric MMP-9 Amplifies Neutrophil Chemotaxis and Enables Functional Studies on MMP-9 Proteoforms.Cells. 2020 Jul 7;9(7):1634. doi: 10.3390/cells9071634. Cells. 2020. PMID: 32645949 Free PMC article.
-
Multidimensional communication in the microenvirons of glioblastoma.Nat Rev Neurol. 2018 Aug;14(8):482-495. doi: 10.1038/s41582-018-0025-8. Nat Rev Neurol. 2018. PMID: 29985475 Free PMC article. Review.
-
Development of a fluorogenic ADAMTS-7 substrate.J Enzyme Inhib Med Chem. 2021 Dec;36(1):2160-2169. doi: 10.1080/14756366.2021.1983808. J Enzyme Inhib Med Chem. 2021. PMID: 34587841 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Miscellaneous

