Prototype of an Interface for Hyphenating Distillation with Gas Chromatography and Mass Spectrometry
- PMID: 28337400
- PMCID: PMC5359752
- DOI: 10.5702/massspectrometry.S0061
Prototype of an Interface for Hyphenating Distillation with Gas Chromatography and Mass Spectrometry
Abstract
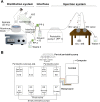
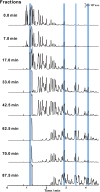
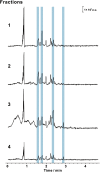
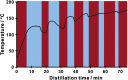
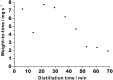
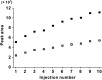
Chemical analysis of complex matrices-containing hundreds of compounds-is challenging. Two-dimensional separation techniques provide an efficient way to reduce complexity of mixtures analyzed by mass spectrometry (MS). For example, gasoline is a mixture of numerous compounds, which can be fractionated by distillation techniques. However, coupling conventional distillation with other separations as well as MS is not straightforward. We have established an automatic system for online coupling of simple microscale distillation with gas chromatography (GC) and electron ionization MS. The developed system incorporates an interface between the distillation condenser and the injector of a fused silica capillary GC column. Development of this multidimensional separation (distillation-GC-MS) was preceded by a series of preliminary off-line experiments. In the developed technique, the components with different boiling points are fractionated and instantly analyzed by GC-MS. The obtained data sets illustrate dynamics of the distillation process. An important advantage of the distillation-GC-MS technique is that raw samples can directly be analyzed without removal of the non-volatile matrix residues that could contaminate the GC injection port and the column. Distilling the samples immediately before the injection to the GC column may reduce possible matrix effects-especially in the early phase of separation, when molecules with different volatilities co-migrate. It can also reduce losses of highly volatile components (during fraction collection and transfer). The two separation steps are partly orthogonal, what can slightly increase selectivity of the entire analysis.
Keywords: distillation; gas chromatography; gasoline; hyphenated techniques; online sample preparation.
Figures


References
-
- 1) J. G. Speight. Handbook of Petroleum Product Analysis. Wiley, Hoboken, 2002.
-
- 2) D. Yergin. The Prize: The Epic Quest for Oil, Money & Power. Free Press, New York, 2008.
-
- 3) A. M. Hochhauser. Gasoline and other motor fuels. Kirk-Othmer Encyclopedia of Chemical Technology 12: 386–435, 2014.
-
- 4) A. Y. El-Naggar, M. M. Al Majthoub. Study the toxic effects of aromatic compounds in gasoline in Saudi Arabia petrol stations. Int. J. Chem. Sci. 11: 106–120, 2013.
-
- 5) J.-Z. Zhang, J. Jin. The influence of common plastics on the identification of gasoline studied by GC-MS. Procedia Eng. 71: 372–376, 2014.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

