Comparative Proteomics Analysis of Human Macrophages Infected with Virulent Mycobacterium bovis
- PMID: 28337427
- PMCID: PMC5343028
- DOI: 10.3389/fcimb.2017.00065
Comparative Proteomics Analysis of Human Macrophages Infected with Virulent Mycobacterium bovis
Abstract
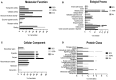
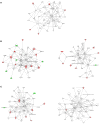
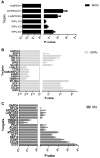
Mycobacterium bovis (M. bovis), the most common pathogens of tuberculosis (TB), is virulent to human and cattle, and transmission between cattle and humans warrants reconsideration concerning food safety and public health. Recently, efforts have begun to analyze cellular proteomic responses induced by Mycobacterium tuberculosis (M. tb). However, the underlying mechanisms by which virulent M. bovis affects human hosts are not fully understood. For the present study, we utilized a global and comparative labeling strategy of isobaric tag for relative and absolute quantitation (iTRAQ) to assess proteomic changes in the human monocyte cell line (THP-1) using a vaccine strain and two virulent strains H37Rv and M. bovis. We measured 2,032 proteins, of which 61 were significantly differentially regulated. Ingenuity Pathway Analysis was employed to investigate the canonical pathways and functional networks involved in the infection. Several pathways, most notably the phagosome maturation pathway and TNF signaling pathway, were differentially affected by virulent strain treatment, including the key proteins CCL20 and ICAM1. Our qRT-PCR results were in accordance with those obtained from iTRAQ. The key enzyme MTHFD2, which is mainly involved in metabolism pathways, as well as LAMTOR2 might be effective upon M. bovis infection. String analysis also suggested that the vacuolar protein VPS26A interacted with TBC1D9B uniquely induced by M. bovis. In this study, we have first demonstrated the application of iTRAQ to compare human protein alterations induced by virulent M. bovis infections, thus providing a conceptual understanding of mycobacteria pathogenesis within the host as well as insight into preventing and controlling TB in human and animal hosts' transmission.
Keywords: M. bovis; M. tb; THP-1 cell; iTRAQ; pathway analysis; proteomics.
Figures




Similar articles
-
Innate cytokine profiling of bovine alveolar macrophages reveals commonalities and divergence in the response to Mycobacterium bovis and Mycobacterium tuberculosis infection.Tuberculosis (Edinb). 2014 Jul;94(4):441-50. doi: 10.1016/j.tube.2014.04.004. Epub 2014 May 5. Tuberculosis (Edinb). 2014. PMID: 24882682
-
Comparative transcriptomic analysis of THP-1-derived macrophages infected with Mycobacterium tuberculosis H37Rv, H37Ra and BCG.J Cell Mol Med. 2021 Nov;25(22):10504-10520. doi: 10.1111/jcmm.16980. Epub 2021 Oct 10. J Cell Mol Med. 2021. PMID: 34632719 Free PMC article.
-
Quantitative proteomic analysis of host responses triggered by Mycobacterium tuberculosis infection in human macrophage cells.Acta Biochim Biophys Sin (Shanghai). 2017 Sep 1;49(9):835-844. doi: 10.1093/abbs/gmx080. Acta Biochim Biophys Sin (Shanghai). 2017. PMID: 28910983
-
Does Mycobacterium bovis persist in cattle in a non-replicative latent state as Mycobacterium tuberculosis in human beings?Vet Microbiol. 2020 Aug;247:108758. doi: 10.1016/j.vetmic.2020.108758. Epub 2020 Jun 21. Vet Microbiol. 2020. PMID: 32768211 Review.
-
Epidemiology of Mycobacterium bovis and Mycobacterium tuberculosis in animals: Transmission dynamics and control challenges of zoonotic TB in Ethiopia.Prev Vet Med. 2018 Oct 1;158:1-17. doi: 10.1016/j.prevetmed.2018.06.012. Epub 2018 Jun 28. Prev Vet Med. 2018. PMID: 30220382 Review.
Cited by
-
Proteogenomics in Aid of Host-Pathogen Interaction Studies: A Bacterial Perspective.Proteomes. 2017 Oct 11;5(4):26. doi: 10.3390/proteomes5040026. Proteomes. 2017. PMID: 29019919 Free PMC article. Review.
-
miR-495 Regulates Cellular Reactive Oxygen Species Levels by Targeting sod2 To Inhibit Intracellular Survival of Mycobacterium tuberculosis in Macrophages.Infect Immun. 2021 Nov 16;89(12):e0031521. doi: 10.1128/IAI.00315-21. Epub 2021 Sep 20. Infect Immun. 2021. PMID: 34543119 Free PMC article.
-
The multivalency game ruling the biology of immunity.Biophys Rev (Melville). 2023 Dec 29;4(4):041306. doi: 10.1063/5.0166165. eCollection 2023 Dec. Biophys Rev (Melville). 2023. PMID: 38505426 Free PMC article. Review.
-
Global proteomics reveals pathways of mesenchymal stem cells altered by Mycobacterium tuberculosis.Sci Rep. 2024 Dec 28;14(1):30677. doi: 10.1038/s41598-024-75722-5. Sci Rep. 2024. PMID: 39730375 Free PMC article.
-
Proteomics and pulse azidohomoalanine labeling of newly synthesized proteins: what are the potential applications?Expert Rev Proteomics. 2018 Jul;15(7):545-554. doi: 10.1080/14789450.2018.1500902. Epub 2018 Jul 23. Expert Rev Proteomics. 2018. PMID: 30005169 Free PMC article. Review.
References
-
- Arbues A., Lugo-Villarino G., Neyrolles O., Guilhot C., Astarie-Dequeker C. (2015). Playing hide-and-seek with host macrophages through the use of mycobacterial cell envelope phthiocerol dimycocerosates and phenolic glycolipids. Front. Cell. Infect. Microbiol. 4:173. 10.3389/fcimb.2014.00173 - DOI - PMC - PubMed
-
- Ayele W. Y., Neill S. D., Zinsstag J., Weiss M. G., Pavlik I. (2004). Bovine tuberculosis: an old disease but a new threat to Africa. Int. J. Tuberculosis Lung Dis. 8, 924–937. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

